UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
(Amendment No. )
Filed by the Registrant ☒
Filed by a Party other than the Registrant ☐
Check the appropriate box:
☐ | Preliminary Proxy Statement |
☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ☒ | Definitive Proxy Statement |
☐ | Definitive Additional Materials |
☐ | Soliciting Material Pursuant to §240.14a-12 |
Nektar Therapeutics
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
☒ | No fee required. |
☐ | Fee paid previously with preliminary materials. |
☐ | Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11 |

NEKTAR THERAPEUTICS
455 Mission Bay Boulevard South
San Francisco, California 94158
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON JUNE 8, 2023June 5, 2024
AT 2:00 P.M. PACIFIC TIME
Dear Stockholder:
You are cordially invited to attend the 20232024 Annual Meeting of Stockholders of Nektar Therapeutics, a Delaware corporation, which will be held by live webcast only. The 20232024 Annual Meeting will be held on Thursday,Wednesday, June 8, 2023,5, 2024 at 2:00 p.m. Pacific Time for the following purposes:
| 1. | To elect |
| 2. | To approve an amendment to our Amended and Restated 2017 Performance Incentive Plan to increase the aggregate number of shares of common stock authorized for issuance under the plan by |
| 3. | To ratify the selection of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, |
| 4. | To approve a non-binding advisory resolution regarding our executive compensation (a “say-on-pay” vote). |
| 5. | To |
These items of business are more fully described in the Proxy Statement accompanying this Notice of Annual Meeting of Stockholders. The record date for the 20232024 Annual Meeting is April 10, 2023.8, 2024. Only stockholders of record at the close of business on that date are entitled to notice of, and to vote at, the 20232024 Annual Meeting or any adjournment thereof.
For the convenience of our stockholders, we have elected to hold the 20232024 Annual Meeting by means of remote communication. The live webcast of the Annual Meeting will begin promptly at 2:00 p.m. Pacific Time.
To participate in the live webcast, please visit www.virtualshareholdermeeting.com/NKTR2023.NKTR2024. You will need the control number included on your Notice of Availability of Proxy Materials, proxy card, or voting instruction form. We encourage you to access the meeting prior to the start time to allow time for check-in procedures. If you experience any technical difficulties during the check-in process or during the meeting, please call the number provided on the meeting website for technical support.
Your vote is very important. Whether or not you participate in the 20232024 Annual Meeting, which will be held by live webcast on the day of the meeting, it is important that your shares be represented. You may vote your proxy on the Internet, by phone or by mail in accordance with the voting instructions in the Notice of Availability of Proxy Materials.
On behalf of the Boardboard of Directors,directors, thank you for your participation in this important annual process.
| | | By Order of the Board of Directors | |
| | | ||
| | | /s/ Mark A. Wilson | |
| | | Mark A. Wilson Senior Vice President, Chief Legal Officer and Secretary | |
San Francisco, California | | | |
April 26, 2024 | | |
YOU ARE CORDIALLY INVITED TO ATTEND THE 20232024 ANNUAL MEETING OF STOCKHOLDERS VIA LIVE WEBCAST ON THE DAY OF THE MEETING. WHETHER OR NOT YOU EXPECT TO ATTEND THE ANNUAL MEETING, PLEASE VOTE ON THE INTERNET, BY PHONE OR BY MAIL AS INSTRUCTED IN THE NOTICE OF AVAILABILITY OF PROXY MATERIALS, AS PROMPTLY AS POSSIBLE IN ORDER TO ENSURE YOUR REPRESENTATION AT THE 20232024 ANNUAL MEETING. EVEN IF YOU HAVE VOTED BY PROXY, YOU MAY STILL VOTE DURING THE LIVE WEBCAST IF YOU ATTEND THE ANNUAL MEETING. PLEASE NOTE, HOWEVER, THAT IF YOUR SHARES ARE HELD OF RECORD BY A BROKER, BANK OR OTHER NOMINEE AND YOU WISH TO VOTE AT THE 20232024 ANNUAL MEETING, YOU MUST OBTAIN A PROXY ISSUED IN YOUR NAME FROM THAT RECORD HOLDER.

NEKTAR THERAPEUTICS
455 Mission Bay Boulevard South
San Francisco, California 94158
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON JUNE 8, 2023June 5, 2024
AT 2:00 P.M. PACIFIC TIME
For the convenience of our stockholders, we have elected to hold the 20232024 Annual Meeting of Stockholders solely by means of remote communication.
WHY AM I RECEIVING THESE MATERIALS?
We sent you a Notice of Availability of Proxy Materials (the “Notice”) because the board of directors of Nektar Therapeutics, a Delaware corporation (“Nektar,” the “Company,” “we” or “us”), is soliciting your proxy to vote at our 20232024 Annual Meeting of Stockholders (the “Annual Meeting”) to be held solely by live webcast on June 8, 20235, 2024 at 2:00 p.m. Pacific Time. There will be no in-person meeting. We invite you to attend the Annual Meeting by live webcast to vote on the proposals described in this proxy statement. However, you do not need to attend the live webcast meeting to vote your shares. Instead, you may vote by proxy over the Internet or by phone or by following the instructions provided in the Notice or, if you requestrequested printed copies of the proxy materials by mail, you may vote by mail. Please visit our website at www.nektar.com for updated information related to the Annual Meeting. As always, we encourage you to vote your shares prior to the Annual Meeting.
The webcast of the Annual Meeting will begin promptly at 2:00 p.m. Pacific Time. To participate in the live webcast, please visit www.virtualshareholdermeeting.com/NKTR2023.NKTR2024. You will need the control number included on your Notice, proxy card, or voting instruction form. We encourage you to access the meeting prior to the start time to allow time for check-in procedures. If you experience any technical difficulties during the check-in process or during the meeting, please call the number provided on the meeting website for technical support.
You may submit a question during the live webcast of the Annual Meeting by visiting www.virtualshareholdermeeting.com/NKTR2023.NKTR2024. We will endeavor to answer as many questions received as time allows that comply with our Annual Meeting rules of conduct. If we receive substantially similar questions, we may group such questions together and provide a single response to avoid repetition. We also reserve the right to exclude questions regarding topics that are not relevant to the meeting matters. Information regarding the rules and procedures for participating in the Annual Meeting will be set forth in our Annual Meeting rules of conduct, which will be available during the meeting on the meeting website.
The Notice was first sent or made available on or about April 28, 202326, 2024 to all stockholders of record entitled to vote at the Annual Meeting.
WHO CAN VOTE AT THE ANNUAL MEETING?
Only stockholders of record at the close of business on April 10, 20238, 2024 will be entitled to vote at the Annual Meeting. On this record date, there were 189,235,157183,624,232 shares of common stock outstanding and entitled to vote.
Stockholder of Record: Shares Registered in Your Name
If, on April 10, 2023,8, 2024, your shares were registered directly in your name with our transfer agent, Computershare Inc., then you are a stockholder of record. The Notice will be sent to you by mail directly by us. As a stockholder of record, you may vote remotely at the Annual Meeting or vote by proxy. Whether or not you plan to attend the Annual Meeting remotely, we urge you to vote on the Internet or by phone as instructed in the Notice or by proxy by mail by requesting a paper copy of the proxy materials as instructed in the Notice to ensure your vote is counted.
1
Beneficial Owner: Shares Registered in the Name of a Broker, Bank or Other Agent
If, on April 10, 2023,8, 2024, your shares were held in an account at a brokerage firm, bank or other agent, then you are the beneficial owner of shares held in “street name” and the Notice is being forwarded to you by that organization. The organization holding your account is considered the stockholder of record for purposes of voting at the Annual Meeting. As a beneficial owner, you have the right to direct your broker, bank or other agent on how to vote the shares in your account. Your brokerage firm, bank or other agent will not be able to vote in the election of directors unless they have your voting instructions, so it is very important that you indicate your voting instructions to the institution holding your shares.
You are also invited to attend the Annual Meeting by live webcast. However, since you are not the stockholder of record, you may not vote your shares remotely at the Annual Meeting by live webcast unless you request and obtain a valid proxy from your broker, bank or other agent.
WHAT AM I VOTING ON?
There are five matters scheduled for a vote:
Proposal 1: To elect twothree directors with terms to expire at the 20262027 Annual Meeting of Stockholders.
Proposal 2: To approve an amendment to our Amended and Restated 2017 Performance Incentive Plan to increase the aggregate number of shares of common stock authorized for issuance under the plan by 12,000,0008,000,000 shares.
Proposal 3: To ratify the selection of Ernst & Young LLP as our independent registered public accounting firm for our fiscal year ending December 31, 2023.2024.
Proposal 4: To approve a non-binding advisory resolution regarding our executive compensation (a “say-on-pay” vote).
HOW ARE PROXY MATERIALS DISTRIBUTED?
Under rules adopted by the Securities and Exchange Commission (“SEC”), we are sending the Notice to our stockholders of record and beneficial owners as of April 10, 2023.8, 2024. Stockholders will have the ability to access the proxy materials, including this proxy statement and our Annual Report on Form 10-K for the fiscal year ended December 31, 2022,2023, on the Internet at www.nektar.com or to request a printed or electronic set of the proxy materials at no charge. Instructions on how to access the proxy materials over the Internet and how to request a printed copy may be found on the Notice.
In addition, any stockholder may request to receive proxy materials in printed form by mail or electronically by email on an ongoing basis. Choosing to receive future proxy materials by email will save us the cost of printing and mailing documents to stockholders and will reduce the impact of annual meetings on the environment. A stockholder who chooses to receive future proxy materials by email will receive an email prior to next year’s annual meeting with instructions containing a link to those materials and a link to the proxy voting website. A stockholder’s election to receive proxy materials by email will remain in effect until the stockholder terminates it.
HOW DO I VOTE?
You may either vote “For” or “Against” or abstain from voting with respect to each nominee to the board of directors. For Proposals 2, 3, and 4 you may vote “For” or “Against” or abstain from voting. For Proposal 5, you may vote for “every year”, “every two years”, “every three years”, or abstain.
The procedures for voting are:
Stockholder of Record: Shares Registered in Your Name
If you are a stockholder of record as of April 10, 2023,8, 2024, you may vote remotely at the Annual Meeting by live webcast, vote by proxy over the Internet or by phone or by following the instructions provided in the Notice or, if you request printed copies of the proxy materials by mail, you may vote by mail. If your proxy is properly
executed in time to be voted at the Annual Meeting, the shares represented by the proxy will be voted in accordance with the instructions you provide. Whether or not you plan to attend the Annual Meeting, we urge you to vote by proxy to ensure your vote is counted. You may still attend the Annual Meeting and vote remotely if you have already voted by proxy.
2
| 1. | To vote during the meeting, attend the Annual Meeting which will be held by live webcast. To attend the live webcast meeting go to www.virtualshareholdermeeting.com/ |
| 2. | To vote on the Internet prior to the Annual Meeting, go to www.proxyvote.com to complete an electronic proxy card. You will be asked to provide the 16-digit control number from the Notice and follow the instructions. Your vote must be received by 11:59 p.m. Eastern Time on June |
| 3. | To vote by phone, request a paper or email copy of the proxy materials by following the instructions on the Notice and call the number provided with the proxy materials to transmit your voting instructions. Your vote must be received by 11:59 p.m. Eastern Time on June |
| 4. | To vote by mail, request a paper copy of the |
Beneficial Owner: Shares Registered in the Name of a Broker, Bank or Other Agent
If you are a beneficial owner of shares registered in the name of your broker, bank or other agent, you should have received a Notice and voting instructions from that organization rather than from us. Simply follow the instructions to ensure that your vote is counted. To vote by live webcast at the Annual Meeting, you must obtain a valid proxy from your broker, bank or other agent. Follow the instructions from your broker, bank or other agent included with the Notice, or contact your broker, bank or other agent.
We provide Internet proxy voting to allow you to vote your shares online, with procedures designed to ensure the authenticity and correctness of your proxy vote instructions. However, please be aware that you must bear any costs associated with your Internet access, such as usage charges from Internet access providers and telephone companies.
HOW MANY VOTES DO I HAVE?
On each matter to be voted upon, you have one vote for each share of common stock you owned as of April 10, 2023.8, 2024
WHAT IS THE QUORUM REQUIREMENT?
A quorum of stockholders is necessary to take any action during the meeting (other than to adjourn the meeting). The presence, by live webcast or by proxy duly authorized, of the holders of a majority of the outstanding shares of stock entitled to vote will constitute a quorum. On April 10, 2023,8, 2024, there were 189,235,157183,624,232 shares outstanding and entitled to vote.
Your shares will be counted towards the quorum only if you submit a valid proxy or vote during the live webcast at the Annual Meeting. Even if your valid proxy card indicates that you abstain from voting or if a broker indicates on a proxy that it lacks discretionary authority to vote your shares on a particular matter, commonly referred to as “broker non-votes,” your shares will still be counted for purposes of determining the presence of a quorum at the Annual Meeting. If there is no quorum, the chairmanchairperson of the Annual Meeting or a majority of the votes present at the Annual Meeting may adjourn the Annual Meeting to another date.
WHAT IF I RETURN A PROXY CARD BUT DO NOT MAKE SPECIFIC CHOICES?
If you are a stockholder of record and you return a proxy card without marking any voting selections, your shares will be voted:
| 1. | Proposal 1: “For” election of the |
| 2. | Proposal 2: “For” the approval of an amendment to the Amended and Restated 2017 Performance Incentive Plan to increase the aggregate number of shares of common stock authorized for issuance under the plan by |
3
| 3. | Proposal 3: “For” the ratification of the Audit Committee’s selection of Ernst & Young LLP as our independent registered public accounting firm for our fiscal year ending December 31, |
| 4. | Proposal 4: “For” the approval of a non-binding advisory resolution regarding our executive compensation (a “say-on-pay” vote). |
If any other matter is properly presented at the meeting, your proxy (one of the individuals named on your proxy card) will vote your shares using histheir best judgment.
If you are a beneficial owner of shares registered in the name of your broker, bank or other agent, your shares are held by your broker, bank or other agent as your nominee (that is, in “street name”) and you will need to obtain a proxy form from the organization that holds your shares and follow the instructions included on that form regarding how to instruct the organization to vote your shares. If you do not give instructions to your broker, bank or other agent, it can vote your shares with respect to “discretionary” items but not with respect to “non-discretionary” items. Discretionary items are proposals considered routine under the rules of various national securities exchanges, and, in the absence of your voting instructions, your broker, bank or other agent may vote your shares held in street name on such proposals. Non-discretionary items are proposals considered non-routine under the rules of various national securities exchanges, and, in the absence of your voting instructions, your broker, bank or other agent may not vote your shares held in street name on such proposals and the shares will be treated as broker non-votes. Proposals 1, 2, 4 and 54 are matters considered non-routine under the applicable rules. If you do not give your broker specific instructions, the broker will not vote your shares on Proposals 1, 2, 4 and 54 and your shares will constitute broker non-votes which will be counted for purposes of determining whether a quorum exists, but will not affect the outcome of these proposals. Proposal 3 involves a mattermatters we believe to be routine and thus if you do not give instructions to your broker, the broker may vote your shares in its discretion on Proposal 3 and therefore no broker non-votes are expected to exist in connection with Proposal 3.
HOW ARE VOTES COUNTED?
Votes will be counted by the inspector of election appointed for the Annual Meeting, with respect to Proposal 1, “For” votes, “Against” votes, abstentions and broker non-votes for each nominee, with respect to ProposalProposals 2 and 4, “For” votes, “Against” votes, abstentions and broker non-votes, with respect to Proposal 5, separate votes for each of “every year”, “every two years” and “every three years”, abstentions and broker non-votes, and with respect to Proposal 3, “For” votes, “Against” votes and abstentions.
WHO WILL SERVE AS INSPECTOR OF ELECTIONS?
A representative of Broadridge Financial Solutions, Inc. will serve as the inspector of elections.
HOW MANY VOTES ARE NEEDED TO APPROVE EACH PROPOSAL?
For Proposal 1 electing twothree members of the board of directors, each director must receive a “For” vote from a majority of the votes cast during the live webcast or by proxy at the Annual Meeting on the election of the director. A majority of the votes cast shall mean that the number of shares voted “For” a director’s election exceeds fifty percent (50%) of the number of the votes cast with respect to that director’s election.
For Proposal 2 approving an amendment to our Amended and Restated 2017 Performance Incentive Plan to increase the aggregate number of shares of common stock authorized for issuance under the plan, the proposal must receive a “For” vote from a majority of the votes cast either during the live webcast or by proxy at the Annual Meeting.
For Proposal 3 ratifying the Audit Committee’s selection of Ernst & Young LLP as our independent registered public accounting firm for our fiscal year ending December 31, 2023,2024, the proposal must receive a “For” vote from a majority of the votes cast either during the live webcast or by proxy at the Annual Meeting.
For Proposal 4 approving the resolution regarding executive compensation, the proposal must receive a “For” vote from a majority of the votes cast either during the live webcast or by proxy at the Annual Meeting.
For Proposal 5 requesting the frequency of our “say-on-pay” vote, the frequency receiving the most votes cast either during the live webcast or by proxy will be considered requested by stockholders.
4
WHO IS PAYING FOR THIS PROXY SOLICITATION?
We will pay for the entire cost of soliciting proxies. In addition to the Notice and the proxy materials, our directors and employees may also solicit proxies during the live webcast, by telephone or by other means of communication. We have retained Georgeson LLC (“Georgeson”) to assist in the distribution of proxy materials and the solicitation of proxies from brokerage firms, fiduciaries, custodians, and other similar organizations representing beneficial owners of shares for the Annual Meeting. We have agreed to pay Georgeson a fee of approximately $15,500 plus customary costs and expenses for these services. We will not pay our directors and employees any additional compensation for soliciting proxies. We may also reimburse brokerage firms, banks and other agents for the cost of forwarding the Notice and any other proxy materials to beneficial owners.
WHAT DOES IT MEAN IF I RECEIVE MORE THAN ONE NOTICE?
If you receive more than one Notice, your shares are registered in more than one name or are registered in different accounts. Please vote by proxy according to each Notice to ensure that all of your shares are voted.
CAN I CHANGE MY VOTE AFTER SUBMITTING MY PROXY?
Yes, you can revoke your proxy at any time before the final vote at the Annual Meeting. If you are a stockholder of record, you may revoke your proxy in any one of three ways:
| 1. | A duly executed proxy card with a later date or time than the previously submitted proxy; |
| 2. | A written notice that you are revoking your proxy to our Secretary, care of Nektar Therapeutics, at 455 Mission Bay Boulevard South, San Francisco, California 94158; or |
| 3. | A later-dated vote on the Internet or by phone or a ballot cast during the live webcast at the Annual Meeting (simply attending the Annual Meeting will not, by itself, revoke your proxy). |
If you are a beneficial owner, you may revoke your proxy by submitting new instructions to your broker, bank or other agent, or if you have received a proxy from your broker, bank or other agent giving you the right to vote your shares at the Annual Meeting, by attending the meeting and voting during the live webcast.
WHEN ARE STOCKHOLDER PROPOSALS DUE FOR NEXT YEAR’S ANNUAL MEETING?
Pursuant to Rule 14a-8 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), some stockholder proposals may be eligible for inclusion in our 20242025 proxy statement. Any such proposal must be submitted in writing by December 29, 2023, to our Secretary, care of Nektar Therapeutics, 455 Mission Bay Boulevard South, San Francisco, California 94158.94158, by December 27, 2024. If we change the date of our 20242025 annual meeting by more than 30 days from the date of the previous year’s annual meeting, the deadline shall be a reasonable time before we begin to print and send our proxy materials.no later than the close of business on the tenth day following the day on which public announcement of the annual meeting date is first made. Stockholders interested in submitting such a proposal are advised to contact knowledgeable counsel with regard to the detailed requirements of the applicable securities laws and our bylaws. The submission of a stockholder proposal does not guarantee that it will be included in our proxy statement.
Alternatively, under our bylaws, if you wish to submit a proposal that is not to be included in next year’s proxy statement or nominate a director, you must provide specific information to us no earlier than March 11, 20247, 2025 and no later than the close of business on April 9, 2024.6, 2025. If we change the date of our 20242025 annual meeting by more than 30 days from the date of the previous year’s annual meeting, the deadline shall be changed to not later than the sixtieth day prior to such annual meeting or the close of business on the tenth day following the day on which public announcement of the annual meeting date is first made, and no earlier than the close of business on the ninetieth day prior to such annual meeting. The public announcement of an adjournment or postponement of the 20242025 annual meeting does not commence a new time period (or extend any time period) for the giving of a stockholder’s notice as described in this proxy statement. You are advised to review our bylaws, which contain additional requirements with respect to advance notice of stockholder proposals and director nominees.
A stockholder’s submission must include certain specific information concerning the proposal or nominee, as the case may be, and information as to the stockholder’s ownership of our common stock. Proposals or nominations not meeting these requirements will not be entertained at any annual meeting.
5
In relation to stockholder proposals and nominations, in certain instances we may exercise discretionary voting authority under proxies held by the board of directors. For instance, if we do not receive a stockholder proposal by April 9, 2024,6, 2025, we may exercise discretionary voting authority under proxies held by the board of directors on such stockholder proposal. If we change the date of our 20232025 annual meeting by more than 30 days from the date of the previous year’s annual meeting, the deadline will change to a reasonable time before we begin to print and send our proxy materials. In addition, even if we are notified of a stockholder proposal within the time requirements discussed above, if the stockholder does not comply with certain requirements of the Exchange Act, we may exercise discretionary voting authority under proxies held by the board of directors on such stockholder proposal if we include advice in our proxy statement on the nature of the matter and how we intend to exercise our discretion to vote on the matter.
WHAT IS “HOUSEHOLDING” AND HOW DOES IT AFFECT ME?
We have adopted a procedure approved by the SEC called “householding.” Under this procedure, stockholders who have the same address may receive only one copy of the Notice, unless one or more of these stockholders notifies us that they wish to receive individual copies of the Notice and, if requested, other proxy materials. This process potentially means extra convenience for stockholders and cost savings for companies.
If you are a beneficial owner of our common stock, once you receive notice from your broker, bank or other agent that they will be householding communications to your address, householding will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in householding and would prefer to receive separate Notices, or other proxy materials, please notify your broker, bank or other agent, direct your written request to Nektar Therapeutics, Secretary, 455 Mission Bay Boulevard South, San Francisco, California 94158 or contact our Secretary at (415) 482-5300. Stockholders who currently receive multiple copies of the Notice or other proxy materials at their address and would like to request householding of their communications should contact their broker, bank or other agent.
HOW CAN I FIND OUT THE RESULTS OF THE VOTING AT THE ANNUAL MEETING?
Preliminary voting results will be announced at the Annual Meeting. Final voting results will be published in a Current Report on Form 8-K filed with the SEC within four business days following the Annual Meeting.
6
ELECTION OF DIRECTORS
Our board of directors is presently comprised of eight (8)seven (7) directors and is divided into three (3) classes. Class I and Class IIIII each currently consist of threetwo directors and Class III currently consists of twothree directors. Each class has a three (3) year term. The three (3) current directors in Class III are Myriam J. Curet, M.D., Karin Eastham,Jeff Ajer, Robert B. Chess and Howard W. Robin,Roy A. Whitfield, whose term expires in 2023.2024. Each of the current directors in Class III was previously elected by the stockholders at the 20202021 Annual Meeting of Stockholders. Ms. Eastham will not seek reelection upon the expiration of her current term at the Annual Meeting. Dr. CuretMr. Ajer, Mr. Chess and Mr. RobinWhitfield have each been nominated for reelection at the Annual Meeting.
Vacancies on the board, including vacancies created by an increase in the number of directors, are filled only by persons elected by a majority of the remaining directors. A director elected by the board to fill a vacancy in a class serves until the earliest of the end of the remaining term of that class, the election and qualification of his or her successor or such director’s death, resignation or removal.
Directors are elected by a majority of the votes cast at the Annual Meeting on the election of directors. A majority of votes cast shall mean that the number of shares voted “For” a director’s election exceeds fifty percent (50%) of the number of votes cast with respect to that director’s election, with votes cast including votes “Against” in each case but excluding abstentions and broker non-votes with respect to that director’s election. Shares represented by executed proxies by stockholders of record will be voted for the election of the twothree nominees named below, unless the “Against” or “Abstain” voting selection has been marked on the proxy card. Neither abstentions nor broker non-votes will have an effect on the outcome of the vote.
Following any election of directors where the number of nominees did not exceed the number of directors to be elected, any incumbent director who was a nominee and who did not receive a majority of votes cast by the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors, shall promptly tender his or her offer of resignation to our board of directors for consideration by our board of directors. A recommendation on whether or not to accept such resignation offer shall be made by the Nominating and Corporate Governance Committee or, if each member of the Nominating and Corporate Governance Committee did not receive the required majority vote or the Nominating and Corporate Governance Committee is otherwise unable to act, a majority of our board of directors shall appoint a special committee of independent directors for such purpose of making a recommendation to our board of directors (the committee with authority to act pursuant to this sentence shall be referred to herein as the “Nominating Committee”). If no independent directors received the required majority vote, our board of directors shall act on the resignation offers.
Within 60 days following certification of the stockholder vote, the Nominating Committee shall consider the resignation offer and recommend to our board of directors the action to be taken with respect to such offer of resignation. Absent a compelling reason for the director to remain on our board of directors, as determined by our board of directors in its business judgment, our board of directors shall accept the resignation offer. Any director who tenders his or her resignation pursuant to this provision shall not participate in the Nominating Committee recommendation or board of directors action regarding whether to accept the resignation offer. Our board of directors shall determine whether to accept the resignation offer and publicly disclose the decision and reasons therefor, by a press release, a filing with the SEC or other broadly disseminated means of communication, within 90 days following certification of the stockholder vote.
If any nominee becomes unavailable for election as a result of an unexpected occurrence, shares that would otherwise be voted for such nominee will be voted for the election of a substitute nominee proposed by the Nominating and Corporate Governance Committee and nominated by the board of directors. Each person nominated for election has agreed to serve if elected. Our management has no reason to believe that any nominee will be unable to serve. If elected at the Annual Meeting, each of the nominees will serve until the earliest of the 20262027 annual meeting of our stockholders, the election and qualification of his or her successor or his or her death, resignation or removal.
7
The following is a brief biography of each nominee.
Robert B. Chess
Robert B. Chess, age 67, is the Chairman of Medicine. Since October 2010, sheour board of directors and has served as a Consulting Professordirector since May 1992. From March 2006 until January 2007, Mr. Chess served as our Acting President and Chief Executive Officer, and from April 1999 to January 2007, served as Executive Chairman. He also served as our Co-Chief Executive Officer from August 1998 to April 2000, as President from December 1991 to August 1998, and as Chief Executive Officer from May 1992 to August 1998. Mr. Chess was previously the co-founder and President of SurgeryPenederm, Inc., a publicly-traded dermatological pharmaceutical company that was sold to Mylan Laboratories. He has held management positions at Stanford University withIntel Corporation and Metaphor Computer Systems (now part of IBM), and was a part time clinical appointment atmember of the Palo Alto Veteran’s Administration Medical Center. She was alsofirst President Bush’s White House staff as a White House Fellow and Associate Director of the White House Office of Economic and Domestic Policy. Mr. Chess serves on the board of directors and is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is Chairman of two private companies: Bighat Biosciences, which does ML-guided biologics design, and Issio Solutions, which is in the labor productivity software field. From 1997 until his retirement in 2009, Mr. Chess served on the board of directors of the Biotechnology Industry Organization (“BIO”). Mr. Chess served as Chairman of BIO’s Emerging Companies Section and Co-Chairman of BIO’s Intellectual Property Committee. Mr. Chess was the initial Chairman of Bio Ventures for Global Health and served on its board through 2022. Mr. Chess serves on the board of directors and is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is currently a member of the faculty atof the UniversityStanford Graduate School of New Mexico for six years prior to joining Stanford UniversityBusiness, where he teaches courses in 2000. Dr. Curetthe MBA program on the healthcare industry and the business opportunity created by aging demographics and increased longevity. Mr. Chess received her M.D.his B.S. degree in Engineering with honors from the California Institute of Technology and an M.B.A. from Harvard Medical School and completed her general surgery residency program at the University of Chicago and her Surgical Endoscopy fellowship at the University of New Mexico.University.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” THE ELECTION OF EACH NAMED
NOMINEE.
8
APPROVAL OF AN AMENDMENT TO OUR AMENDED AND RESTATED 2017 PERFORMANCE INCENTIVE PLAN
At the Annual Meeting, our stockholders will be asked to approve an amendment to the Nektar Therapeutics Amended and Restated 2017 Performance Incentive Plan (the “2017 Plan” and as amended, the “Amended 2017 Plan”) to increase the authorized shares under the Amended 2017 Plan by 12,000,0008,000,000 shares. The 2017 Plan was originally approved by our board of directors on March 28, 2017, subject to stockholder approval which was received on June 14, 2017, and was previously amended on June 26, 2018, June 17, 2020, June 10, 2021, June 8, 2022 and June 8, 2022.2023. Our board of directors approved the Amended 2017 Plan on March 29, 2023,27, 2024, subject to stockholder approval at the Annual Meeting.
Prior to the adoption of the 2017 Plan, the Company maintained the Nektar Therapeutics 2012 Plan, as amended (the “2012 Plan”), the Nektar Therapeutics 2008 Equity Incentive Plan, as amended (the “2008 Plan”), the Nektar Therapeutics 2000 Equity Incentive Plan, as amended (the “2000 Plan”), and the Nektar therapeutics 2000 Non-Officer Equity Incentive Plan, as amended (the (“2000 Non-Officer Plan”). The 2000 Non-Officer Plan together with the 2012 Plan, the 2008 Plan and 2000 Plan, are referred to as the “Prior Plans.” Following stockholder approval of the 2017 Plan, no further awards were made under the Prior Plans. As of April 3, 2023, 21,146,3968, 2024, 26,665,748 shares were subject to outstanding stock options and restricted stock units granted under the 2017 Plan and 3,457,77710,182,402 shares remained available for future grants under the 2017 Plan. In addition, under the terms of the 2017 Plan, the reserve pool under the 2017 Plan may be increased by shares subject to awards granted under the Prior Plans that were outstanding as of December 31, 2017 in the event that such awards expire, or for any reason are cancelled or terminated, without being exercised. As of April 3, 2023, 1,397,9868, 2024, 0 shares remained subject to outstanding stock options and restricted stock units granted under the Prior Plans. If stockholders approve the amendment to the 2017 Plan, as of April 3, 2023,8, 2024, the number of shares available for future awards under the Amended 2017 Plan will increase by 12,000,0008,000,000 shares to 15,457,77718,182,402 shares.
Additional Information on Outstanding Awards and Available Shares under the 2017 Plan and the Prior Plans
The following provides additional information on the total equity compensation awards outstanding and available shares.
| | | As of April 8, 2024 | |
Shares Outstanding and Available for Grant under the 2017 Plan and the Prior Plans | | | |
Total shares subject to outstanding stock options | | | |
Total shares subject to outstanding deferred restricted stock, restricted stock units, and performance restricted stock units | | | |
Weighted-average exercise price of outstanding stock options under all stock incentive plans | | | $ |
Weighted-average remaining contractual life of outstanding stock options (years) | | | |
Total shares available for grant under all stock incentive plans but not yet granted(1) | | |
| (1) | Excludes shares available for purchase under ESPP |
Based solely on the closing price of our common stock as reported by the NASDAQ Global SelectNasdaq Capital Market on April 3, 20238, 2024 and the maximum number of shares that would have been available for awards as of such date, taking into account the proposed increase described herein, the maximum aggregate market value of the common stock that could potentially be issued under the Amended 2017 Plan is $11,284,177.$23,818,947.
Given the limited number of shares that currently remain available under the 2017 Plan, our board of directors and management believe it is important that this amendment be approved in order to maintain the Company’s ability to grant stock-based awards to retain employees and continue to provide them with strong incentives to contribute to the Company’s future success. The Company believes that incentives and stock-based awards focus employees on the objective of creating stockholder value and promoting the success of the Company, and that incentive compensation plans like the 2017 Plan are an important attraction, retention and motivation tool for participants in the plan.
9
All members of the board of directors and all of the Company’s executive officers will be eligible for awards under the Amended 2017 Plan and thus have a personal interest in the approval of the amendment to the 2017 Plan.
Stockholders are requested in this Proposal 2 to approve the amendment to the 2017 Plan. Approval of the amendment to the 2017 Plan requires the affirmative vote of a majority of the votes cast, in person during the live webcast or by proxy, and entitled to vote at the Annual Meeting. Abstentions and broker non-votes to the extent applicable, are not included in the tabulation of the voting results and therefore will not have an effect on the outcome of the vote.vote on this proposal. If stockholders do not approve this amendment, the 2017 Plan will continue in accordance with its current terms.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 2.
The essential features of the 2017 Plan, as proposed to be amended, are outlined below:
The principal terms of the Amended 2017 Plan are summarized below. The following summary is qualified in its entirety by the full text of the 2017 Plan and the amendment to the 2017 Plan, which appears as Exhibit A to this proxy statement.
Purpose. The purpose of the Amended 2017 Plan is to promote the success of the Company and the interests of our stockholders by providing an additional means for us to attract, motivate, retain and reward directors, officers, employees and other eligible persons through the grant of awards. Equity-based awards are also intended to further align the interests of award recipients and our stockholders.
Administration. Our board of directors or one or more committees appointed by our board of directors will administer the Amended 2017 Plan. Our board of directors has delegated general administrative authority for the Amended 2017 Plan to the organization and compensation committee of our board of directors. The organization and compensation committee may delegate some or all of its authority with respect to the Amended 2017 Plan to another committee of directors, and certain limited authority to grant awards to employees may be delegated to one or more officers of the Company. (The appropriate acting body, be it the board of directors, a committee within its delegated authority, or an officer within his or her delegated authority, is referred to in this proposal as the “Administrator”).
The Administrator has broad authority under the Amended 2017 Plan with respect to award grants including, without limitation, the authority:
to select participants and determine the type(s) of award(s) that they are to receive;
to determine the number of shares that are to be subject to awards and the terms and conditions of awards, including the price (if any) to be paid for the shares or the award;
to cancel, modify, or waive the Company’s rights with respect to, or modify, discontinue, suspend, or terminate any or all outstanding awards, subject to any required consents;
to accelerate or extend the vesting or exercisability or extend the term of any or all outstanding awards;
subject to the other provisions of the Amended 2017 Plan, to make certain adjustments to an outstanding award and to authorize the termination, conversion, succession or substitution of an award; and
to allow the purchase price of an award or shares of the Company’s common stock to be paid in the form of cash, check, or electronic funds transfer, by the delivery of already-owned shares of the Company’s common stock or by a reduction of the number of shares deliverable pursuant to the award, by services rendered by the recipient of the award, by notice and third party payment or cashless exercise on such terms as the Administrator may authorize, or any other form permitted by law.
No Repricing. In no case (except due to an adjustment to reflect a stock split or other events referred to under “Adjustments” below, or any repricing that may be approved by stockholders) will the Administrator (1) amend an outstanding stock option or stock appreciation right to reduce the exercise price or base price of the award, (2) cancel, exchange, or surrender an outstanding stock option or stock appreciation right in exchange for cash or other awards for the purpose of repricing the award, or (3) cancel, exchange, or surrender an outstanding stock option or stock appreciation right in exchange for an option or stock appreciation right with an exercise or base price that is less than the exercise or base price of the original award.
10
Eligibility. Persons eligible to receive awards under the Amended 2017 Plan include officers or employees of the Company or any of its subsidiaries, directors of the Company or any of its subsidiaries, and certain consultants and advisors to the Company or any of its subsidiaries. As of April 3, 2023,8, 2024, approximately 209138 employees (including executive officers) and sevensix non-employee directors would be eligible to participate in the 2017 Plan.
Authorized Shares; Limits on Awards. Subject to the adjustment provisions included in the 2017 Plan, the maximum number of shares of the Company’s common stock initially available for issuance pursuant to awards under the 2017 Plan equaled 8,300,000 shares of the Company’s common stock (reduced by the number of shares of common stock subject to awards granted under the 2012 Plan on or after March 31, 2017 and prior to the adoption of the 2017 Plan), which was increased to 19,200,000 by stockholder approval in June 2018, was increased to 29,200,000 by stockholder approval in June 2020,was increased to 34,200,000 by stockholder approval in June 2021, and was increased to 39,200,000 by stockholder approval in June 2022.2022, and was increased to 51,200,000 by stockholder approval in June 2023. The proposed amendment to the 2017 Plan would increase the total available shares to 51,200,000.59,200,000. Shares issued in respect of any “full-value award” granted under the Amended 2017 Plan will be counted against the share limit described in the preceding sentence as 1.50 shares for every one share actually issued in connection with the award. For example, if the Company granted 100 restricted stock units under the Amended 2017 Plan, 150 shares would be charged against the share limit with respect to that award. For this purpose, a “full-value award” generally means any award granted under the plan other than a stock option or stock appreciation right.
The following other limits are also contained in the Amended 2017 Plan:
the maximum number of shares that may be delivered pursuant to options qualified as incentive stock options granted under the plan is 51,200,000;59,200,000;
the maximum number of shares subject to options and stock appreciation rights that are granted during any calendar year to any individual under the plan is 3,000,000 shares;
“performance-based awards” under Section 5.2 of the Amended 2017 Plan granted to a participant in any one calendar year will not provide for payment of more than (1) in the case of awards payable only in cash and not related to shares, $5,000,000, and (2) in the case of awards related to shares (and in addition to options and stock appreciation rights which are subject to the limit referred to above), 3,000,000 shares; and
the aggregate value of cash compensation and the grant date fair value (computed in accordance with generally accepted accounting principles) of shares of common stock that may be paid or granted during any calendar year to any non-employee director shall not exceed $1,200,000 for existing non-employee directors and $2,200,000 for new non-employee directors.
Except as described in the next sentence, shares that are subject to or underlie awards which expire or for any reason are cancelled or terminated, are forfeited, fail to vest, or for any other reason are not paid or delivered under the Amended 2017 Plan or the Prior Plans will again be available for subsequent awards under the Amended 2017 Plan (with any such shares subject to full-value awards increasing the Amended 2017 Plan’s share limit based on the full-value award ratio described above or, in the case of an award granted under a Prior Plan, the full-value award ratio set forth in such Prior Plan). Shares that are exchanged by a participant or withheld by the Company to pay the exercise price of an award granted under the Amended 2017 Plan, as well as any shares exchanged or withheld to satisfy the tax withholding obligations related to any award, will not be available for subsequent awards under the Amended 2017 Plan. To the extent that an award granted under the Amended 2017 Plan or a Prior Plan is settled in cash or a form other than shares, the shares that would have been delivered had there been no such cash or other settlement will again be available for subsequent awards under the Amended 2017 Plan (with any such shares subject to full-value awards increasing the Amended 2017 Plan’s share limit based on the full-value award ratio described above or, in the case of an award granted under a Prior Plan, the full-value award ratio set forth in such Prior Plan). In the event that shares are delivered in respect of a dividend equivalent right, the actual number of shares delivered with respect to the award shall be counted against the share limits of the Amended 2017 Plan. (For purposes of clarity, if 1,000 dividend equivalent rights are granted and outstanding when the Company pays a dividend, and 50 shares are delivered in payment of those rights with respect to that dividend, 75 shares (after adjustment for the full-value award share counting ratio described above) shall be counted against the share limits of the plan.) To the extent that shares are
11
delivered pursuant to the exercise of a stock appreciation right or stock option, the number of underlying shares
as to which the exercise related shall be counted against the applicable share limits, as opposed to only counting the shares issued. (For purposes of clarity, if a stock appreciation right relates to 100,000 shares and is exercised at a time when the payment due to the participant is 15,000 shares, 100,000 shares shall be charged against the applicable share limits with respect to such exercise.) In addition, the Amended 2017 Plan generally provides that shares issued in connection with awards that are granted by or become obligations of the Company through the assumption of awards (or in substitution for awards) in connection with an acquisition of another company will not count against the shares available for issuance under the Amended 2017 Plan. The Company may not increase the applicable share limits of the Amended 2017 Plan by repurchasing shares of common stock on the market (by using cash received through the exercise of stock options or otherwise).
Types of Awards. The Amended 2017 Plan authorizes stock options, stock appreciation rights, stock bonuses, restricted stock, performance stock, stock units, phantom stock or similar rights to purchase or acquire shares, whether at a fixed or variable price or ratio related to the common stock, upon the passage of time, the occurrence of one or more events or the satisfaction of performance criteria or other conditions, awards of any similar securities with a value derived from the value of or related to the common stock and/or returns thereon, or cash awards. The Amended 2017 Plan retains flexibility to offer competitive incentives and to tailor benefits to specific needs and circumstances. Awards granted under the Amended 2017 Plan will be subject to such terms and conditions as established by the Administrator and set forth in the underlying award agreement, including terms relating to the treatment of an award upon a termination of employment. Any award may be paid or settled in cash.
A stock option is the right to purchase shares of the Company’s common stock at a future date at a specified price per share (the “exercise price”). The per share exercise price of an option may not be less than the fair market value of a share of the Company’s common stock on the date of grant. The maximum term of an option is eight years from the date of grant. An option may either be an incentive stock option or a nonqualified stock option. Incentive stock option benefits are taxed differently from nonqualified stock options, as described under “Federal Income Tax Consequences of Awards Under the Amended 2017 Plan” below. Incentive stock options are also subject to more restrictive terms and are limited in amount by the U.S. Internal Revenue Code of 1986, as amended (the “Code”) and the Amended 2017 Plan. Incentive stock options may only be granted to employees of the Company or a subsidiary.
A stock appreciation right is the right to receive payment of an amount equal to the excess of the fair market value of share of the Company’s common stock on the date of exercise of the stock appreciation right over the base price of the stock appreciation right. The base price will be established by the Administrator at the time of grant of the stock appreciation right and may not be less than the fair market value of a share of the Company’s common stock on the date of grant. Stock appreciation rights may be granted in connection with other awards or independently. The maximum term of a stock appreciation right is eight years from the date of grant.
Performance-Based Awards. The Administrator may grant performance-based awards under the Amended 2017 Plan. Performance-based awards are in addition to any of the other types of awards that may be granted under the Amended 2017 Plan. Performance-based awards may be in the form of restricted stock, performance stock, stock units, other rights, or cash bonus opportunities.
The vesting or payment of performance-based awards may depend on the absolute or relative performance of the Company on a consolidated, subsidiary, segment, division, or business unit basis. The Administrator will establish the criterion or criteria and target(s) on which performance will be measured. The criteria that the Administrator may use for this purpose may include, without limitation, any one or more of the following: earnings per share; cash flow (which means cash and cash equivalents derived from either net cash flow from operations or net cash flow from operations, financing and investing activities); working capital; stock price; total stockholder return; revenue; gross profit; operating income; net earnings (before or after interest, taxes, depreciation and/or amortization); gross margin; operating margin; net margin; return on equity or on assets or on net investment; cost containment or reduction; regulatory submissions or approvals; manufacturing production; completion of strategic partnerships; research milestones; any other measure selected by the Administrator or any combination thereof. As applicable, these terms are used as applied under generally accepted accounting principles or in the financial reporting of the Company or of its subsidiaries. The applicable performance goals may be applied on a pre- or post-tax basis and may be adjusted to include or exclude determinable components
12
of any performance goal, including, without limitation, foreign exchange gains and losses, asset write-downs,
acquisitions and divestitures, change in fiscal year, unbudgeted capital expenditures, special charges such as restructuring or impairment charges, debt refinancing costs, extraordinary or noncash items, unusual, infrequently occurring, nonrecurring or one-time events affecting the Company or its financial statements or changes in law or accounting principles.
The performance measurement period with respect to an award may range from three months to ten years. Performance-based awards may be paid in stock or in cash (in either case, subject to the limits described under the heading “Authorized Shares; Limits on Awards” above). The Administrator has discretion to determine the performance target or targets and any other restrictions or other limitations of performance-based awards and may reserve discretion to reduce payments below maximum award limits.
Dividend Equivalents; Deferrals. The Administrator may provide for the deferred payment of awards, and may determine the other terms applicable to deferrals. The Administrator may provide that awards under the Amended 2017 Plan (other than options or stock appreciation rights), and/or deferrals, earn dividends or dividend equivalents based on the amount of dividends paid on outstanding shares of common stock, provided that as to any dividends or dividend equivalent rights granted in connection with an award granted under the Amended 2017 Plan that is subject to vesting requirements, no dividends or dividend equivalent payments will be made unless the related vesting conditions of the award are satisfied.
Award Agreements. Each award shall be evidenced by either (1) a written award agreement in a form approved by the Administrator and executed by the Company by an officer duly authorized to act on its behalf, or (2) an electronic notice of award grant in a form approved by the Administrator. The award agreement shall set forth the material terms and conditions of the award as established by the Administrator consistent with the express limitations of the Amended 2017 Plan. Notwithstanding anything in the Amended 2017 Plan to the contrary, the Administrator may approve an award agreement that, upon the termination of a participant’s employment or service, provides that, or may, in its sole discretion based on a review of all relevant facts and circumstances, otherwise take action regarding an award agreement such that (i) any or all outstanding stock options and stock appreciation rights will become exercisable in part or in full, (ii) all or a portion of the restriction or vesting period applicable to any outstanding award will lapse, (iii) all or a portion of the performance measurement period applicable to any outstanding award will lapse and (iv) the performance goals applicable to any outstanding award (if any) will be deemed to be satisfied at the target, maximum or any other interim level.
Assumption and Termination of Awards. Generally, and subject to limited exceptions set forth in the Amended 2017 Plan, upon the occurrence of a “change in control,” as defined in the Amended 2017 Plan, the Administrator may provide for the cash payment in settlement of, or for the termination, assumption, substitution or exchange of any or all outstanding awards granted under the Amended 2017 Plan. To the extent the administrator does not provide for the assumption, substitution or other continuation of the awards, then all awards then-outstanding under the Amended 2017 Plan will become fully vested or paid, as applicable, and will terminate or be terminated in such circumstances, provided that the holder of a stock option or stock appreciation right would be given reasonable advance (but no more than ten days’) notice of the impending termination and a reasonable opportunity to exercise his or her vested stock option or stock appreciation right (after giving effect to any accelerated vesting required in the circumstances) in accordance with their terms before the termination of such awards. The Administrator also has the discretion to establish other change in control provisions with respect to awards granted under the Amended 2017 Plan. For example, the Administrator could provide for the acceleration of vesting or payment of an award in connection with a change in control and provide that any such acceleration shall be automatic upon the occurrence of any such event, including a termination of employment within a limited period of time following a corporate transaction.
Transfer Restrictions. Subject to certain exceptions contained in the Amended 2017 Plan, awards under the Amended 2017 Plan generally are not transferable by the recipient other than by will or the laws of descent and distribution and are generally exercisable, during the recipient’s lifetime, only by the recipient. Any amounts payable or shares issuable pursuant to an award generally will be paid only to the recipient or the recipient’s beneficiary or representative. The Administrator has discretion, however, to establish written conditions and procedures for the transfer of awards to other persons or entities, provided that such transfers comply with
13
applicable federal and state securities laws and are not made for value (other than nominal consideration, settlement of marital property rights, or for interests in an entity in which more than 50% of the voting securities are held by the award recipient or by the recipient’s family members).
Adjustments. As is customary in incentive plans of this nature, each share limit and the number and kind of shares available under the Amended 2017 Plan and any outstanding awards, as well as the exercise or purchase prices of awards, and performance targets under certain types of performance-based awards, are subject to adjustment in the event of certain reorganizations, mergers, combinations, recapitalizations, stock splits, stock dividends, or other similar events that change the number or kind of shares outstanding, and extraordinary dividends or distributions of property to the stockholders.
No Limit on Other Authority. The Amended 2017 Plan does not limit the authority of the board of directors or any committee to grant awards or authorize any other compensation, with or without reference to the Company’s common stock, under any other plan or authority.
Termination of or Changes to the Amended 2017 Plan. The board of directors may amend or terminate the Amended 2017 Plan at any time and in any manner. Stockholder approval for an amendment will be required only to the extent then required by applicable law or any applicable listing agency or required under Sections 422 or 424 of the Code to preserve the intended tax consequences of the plan. Unless terminated earlier by the board of directors, the authority to grant new awards under the Amended 2017 Plan will terminate on March 27, 2027. Outstanding awards, as well as the Administrator’s authority with respect thereto, generally will continue following the expiration or termination of the plan. Generally speaking, outstanding awards may be amended by the Administrator (except for a repricing), but the consent of the award holder is required if the amendment (or any plan amendment) materially and adversely affects the holder.
Clawback Policy. The awards under the Amended 2017 Plan are subject to the terms of the Company’s clawback policyNektar Therapeutics Compensation Recovery Policy as it may be in effect from time to time.
Federal Income Tax Consequences of Awards under the Amended 2017 Plan
The U.S. federal income tax consequences of the Amended 2017 Plan under current federal law, which is subject to change, are summarized in the following discussion of the general tax principles applicable to the Amended 2017 Plan. This summary is not intended to be exhaustive and, among other considerations, does not describe the deferred compensation provisions of Section 409A of the Code to the extent an award is subject to and does not satisfy those rules, nor does it describe state, local, or international tax consequences.
With respect to nonqualified stock options, the Company is generally entitled to deduct, except to the extent limited by Section 162(m) of the Code, and the participant recognizes taxable income in an amount equal to the difference between the option exercise price and the fair market value of the shares at the time of exercise. With respect to incentive stock options, the Company is generally not entitled to a deduction nor does the participant recognize income at the time of exercise, although if the participant is subject to the U.S. federal alternative minimum tax, the difference between the option exercise price and the fair market value of the shares at the time of exercise is includible for purposes of such alternative minimum tax. If the shares acquired by exercise of an incentive stock option are held for at least two years from the date the option was granted and one year from the date it was exercised, any gain or loss arising from a subsequent disposition of those shares will be taxed as long-term capital gain or loss, and the Company will not be entitled to any deduction. If, however, such shares are disposed of within the above-described period, then in the year of that disposition the participant will recognize compensation taxable as ordinary income equal to the excess of the lesser of (i) the amount realized upon that disposition and (ii) the excess of the fair market value of those shares on the date of exercise over the purchase price, and the Company will be entitled to a corresponding deduction, except to the extent limited by Section 162(m) of the Code.
The current federal income tax consequences of other awards authorized under the Amended 2017 Plan generally follow certain basic patterns: nontransferable restricted stock subject to a substantial risk of forfeiture results in income recognition equal to the excess of the fair market value over the price paid (if any) only at the time the restrictions constituting a substantial risk of forfeiture lapse (unless the recipient elects to accelerate recognition as of the date of grant); bonuses, restricted stock units, stock appreciation rights, cash and stock-based performance awards, dividend equivalents, stock units, and other types of awards are generally subject to tax at the time of payment; and compensation otherwise effectively deferred is taxed when paid. In
14
each of the foregoing cases, the Company will generally have a corresponding deduction at the time the participant recognizes income, except to the extent limited by Section 162(m) of the Code.
If an award is accelerated under the Amended 2017 Plan in connection with a “change in control” (as defined in the Amended 2017 Plan), the Company may not be permitted to deduct the portion of the
compensation attributable to the acceleration (“parachute payments”) if it exceeds certain threshold limits under Section 280G of the Code (and certain related excise taxes may be triggered). Furthermore, Section 162(m) of the Code limits to $1 million the amount that a publicly held corporation is allowed each year to deduct for compensation paid to the corporation’s “covered employees.” “Covered employees” include the corporation’s chief executive officer, chief financial officer and three next most highly compensated executive officers. If an individual is determined to be a covered employee for any year beginning after December 31, 2017, then that individual will continue to be a covered employee for future years, regardless of changes in the individual’s compensation or position. Beginning on or after January 1, 2027, the American Rescue Plan Act of 2021 (the “ARPA”) expands the applicability of Section 162(m) of the Code to also include the next five highest paid corporate officers so that the total number of covered employees subject to the $1 million deduction limitation will at least be 10.
New Plan Benefits
The Company has not approved any awards that are conditioned upon stockholder approval of the amendment to the 2017 Plan. The Administrator has the discretion to grant awards under the Amended 2017 Plan and, therefore, it is not possible as of the date of this proxy statement to determine future awards that will be received by the Company’s named executive officers or others under the Amended 2017 Plan. Accordingly, in lieu of providing information regarding benefits that will be received under the Amended 2017 Plan, the following table provides information concerning the benefits that were received by the following person and groups during 2022:2023: each named executive officer; all named executive offers,officers, as a group; all current directors who are not named executive officers, as a group; and all current employees who are not named executive officers, as a group.
Name and Position | | | Stock Options | | | Restricted Stock Units | ||||||
| | | Number of Shares (#) | | | Average Exercise Price ($) | | | Number of Units (#) | | | Dollar Value ($)(1) | |
Howard W. Robin President and Chief Executive Officer | | | 1,642,500 | | | 4.91 | | | 821,250 | | | 4,032,338 |
Jillian B. Thomsen Chief Financial Officer and Chief Accounting Officer | | | 663,750 | | | 4.91 | | | 331,876 | | | 1,629,511 |
Mark A. Wilson Chief Legal Officer | | | 663,750 | | | 4.91 | | | 331,876 | | | 1,629,511 |
Jonathan Zalevsky, Ph.D. Chief Research and Development Officer | | | 540,000 | | | 4.91 | | | 270,000 | | | 1,325,700 |
Named Executive Officer Group (4 persons) | | | 3,510,000 | | | 4.91 | | | 1,755,002 | | | 8,617,060 |
Non-Executive Director Group (7 persons other than Mr. Robin) | | | 142,800 | | | 3.47 | | | 71,400 | | | 247,758 |
Employee Group (other than named executive officers) (approximately 209 persons)* | | | 6,275,750 | | | 4.76(2) | | | 5,309,176 | | | 19,410,855 |
| | | Stock Options | | | Restricted Stock Units | |||||||
| Name and Position | | | Number of Shares (#) | | | Average Exercise Price ($) | | | Number of Units (#) | | | Dollar Value ($)(1) |
Howard W. Robin President and Chief Executive Officer | | | 2,600,000 | | | 0.50 | | | — | | | — |
Sandra Gardiner(2) Interim Chief Financial Officer | | | — | | | — | | | — | | | — |
Mark A. Wilson Chief Legal Officer | | | 650,000 | | | 0.50 | | | — | | | — |
Jonathan Zalevsky, Ph.D. Chief Research and Development Officer | | | 500,000 | | | 0.50 | | | — | | | — |
| Named Executive Officer Group (4 persons) | | | 3,750,000 | | | 0.50(3) | | | — | | | — |
| Non-Executive Director Group (6 persons other than Mr. Robin) | | | 510,000 | | | 0.68(3) | | | — | | | — |
| Employee Group (other than named executive officers) (approximately 133 persons)* | | | 7,577,650 | | | 0.51(3) | | | 126,000 | | | 139,014(4) |
| * | As of April |
| (1) | The valuation of stock awards is based on the grant date fair value computed in accordance with FASB ASC Topic 718. For a discussion of the assumptions used in calculating these values, see Note |
| (2) | Ms. Gardiner was appointed our Interim Chief Financial Officer as of April 17, 2023. Ms. Gardiner is a partner at FLG Partners, LLC and provides services as Interim Chief Financial Officer as an outside consultant pursuant to a consulting agreement between the Company and FLG Partners, LLC. Ms. Gardiner did not receive any equity grant award in 2023. |
| (3) | Represents the weighted average exercise price for the group. |
| Represents the aggregate grant date fair value for the group. |
15
Equity Compensation Plan Information
The following table presents aggregate summary information as of December 31, 2022,2023, regarding the common stock that may be issued upon the exercise of options and rights under all of our existing equity compensation plans:
Plan Category | | | Number of Securities to be Issued Upon Exercise of Outstanding Options & Vesting of RSUs (a) | | | Weighted-Average Exercise Price of Outstanding Options (b) | | | Number of Securities Remaining Available for Issuance Under Amended 2017 Plan (Excluding Securities Reflected in Column(a)) (c) |
Equity compensation plans approved by security holders(3) | | | 23,589,000 | | | $16.40 | | | 2,973,000 |
Equity compensation plans not approved by security holders | | | 0 | | | $0 | | | 0 |
Total | | | 23,589,000 | | | $16.40 | | | 2,973,000 |
| Plan Category | | | Number of Securities to be Issued Upon Exercise of Outstanding Options & Vesting of RSUs (a) | | | Weighted-Average Exercise Price of Outstanding Options (b) | | | Number of Securities Remaining Available for Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column(a)) (c) |
| Equity compensation plans approved by security holders | | | 27,246,000 | | | $8.26 | | | 10,101,000 |
| Equity compensation plans not approved by security holders | | | 0 | | | $0 | | | 0 |
| Total | | | 27,246,000 | | | $8.26 | | | 10,101,000 |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 2 FOR APPROVAL OF THE AMENDMENT TO OUR AMENDED AND RESTATED 2017 PERFORMANCE INCENTIVE
PLAN AS DESCRIBED ABOVE AND SET FORTH IN EXHIBIT A HERETO.
16
RATIFICATION OF SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Audit Committee of the board of directors has selected Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2023,2024, and has further directed that management submit the selection of our independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. Ernst & Young LLP has audited our consolidated financial statements since our inception. Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither our bylaws nor other governing documents or law require stockholder ratification of the selection of Ernst & Young LLP as our independent registered public accounting firm. However, the Audit Committee is submitting the selection of Ernst & Young LLP to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Audit Committee will reconsider whether or not to retain Ernst & Young LLP. Even if the selection is ratified, the Audit Committee, in its discretion, may direct the appointment of a different independent registered public accounting firm at any time during the year if the committee determines that such a change would be in our best interests and our stockholders’ best interest.
The affirmative vote of the holders of a majority of the votes cast during the live webcast or by proxy at the Annual Meeting will be required to ratify the selection of Ernst & Young LLP for our fiscal year ending December 31, 2023.2024. Abstentions are treated as shares represented during the live webcast or by proxy and entitled to vote at the Annual Meeting and, therefore,broker non-votes, if any, will have no effect on the effectoutcome of athe vote against the ratification of Ernst & Young LLP as our independent registered public accounting firm.on this proposal. No broker non-votes are expected to exist in connection with this proposal.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 3.
17
Robert B. Chess
Robert B. Chess, age 67, is advisory in nature and therefore will not bind us to take any particular action,the Chairman of our board of directors and has served as a director since May 1992. From March 2006 until January 2007, Mr. Chess served as our Acting President and Chief Executive Officer, and from April 1999 to January 2007, served as Executive Chairman. He also served as our Co-Chief Executive Officer from August 1998 to April 2000, as President from December 1991 to August 1998, and as Chief Executive Officer from May 1992 to August 1998. Mr. Chess was previously the co-founder and President of Penederm, Inc., a publicly-traded dermatological pharmaceutical company that was sold to Mylan Laboratories. He has held management positions at Intel Corporation and Metaphor Computer Systems (now part of IBM), and was a member of the first President Bush’s White House staff as a White House Fellow and Associate Director of the White House Office of Economic and Domestic Policy. Mr. Chess serves on the board of directors and is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is Chairman of two private companies: Bighat Biosciences, which does ML-guided biologics design, and Issio Solutions, which is in the labor productivity software field. From 1997 until his retirement in 2009, Mr. Chess served on the board of directors of the Biotechnology Industry Organization (“BIO”). Mr. Chess served as Chairman of BIO’s Emerging Companies Section and Compensation Committee intend to carefully considerCo-Chairman of BIO’s Intellectual Property Committee. Mr. Chess was the stockholder vote resultinginitial Chairman of Bio Ventures for Global Health and served on its board through 2022. Mr. Chess serves on the board of directors and is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is currently a member of the faculty of the Stanford Graduate School of Business, where he teaches courses in the MBA program on the healthcare industry and the business opportunity created by aging demographics and increased longevity. Mr. Chess received his B.S. degree in Engineering with honors from the proposalCalifornia Institute of Technology and an M.B.A. from Harvard University.
Roy A. Whitfield
Roy A. Whitfield, age 70, has served as our director since August 2000 and as Lead Independent Director since January 2019. Mr. Whitfield is the former Chairman of the Board and Chief Executive Officer of Incyte Corporation (“Incyte”), a drug discovery and development company he co-founded in making1991. From January 1993 to November 2001, Mr. Whitfield served as its Chief Executive Officer and from November 2001 until June 2003 as its Chairman. He also served as a director of Incyte from 1991 to January 2014. From 1984 to 1989, Mr. Whitfield held senior operating and business development positions with Technicon Instruments Corporation (“Technicon”), a medical instrumentation company, and its predecessor company, Cooper Biomedical, Inc., a biotechnology and medical diagnostics company. Prior to his work at Technicon, Mr. Whitfield spent seven years with the Boston Consulting Group’s international consulting practice. Mr. Whitfield received a B.S. in mathematics from Oxford University and an M.B.A. from Stanford University.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” THE ELECTION OF EACH NAMED
NOMINEE.
8
APPROVAL OF AN AMENDMENT TO OUR AMENDED AND RESTATED 2017 PERFORMANCE INCENTIVE PLAN
At the Annual Meeting, our stockholders will be asked to approve an amendment to the Nektar Therapeutics Amended and Restated 2017 Performance Incentive Plan (the “2017 Plan” and as amended, the “Amended 2017 Plan”) to increase the authorized shares under the Amended 2017 Plan by 8,000,000 shares.The 2017 Plan was originally approved by our board of directors on March 28, 2017, subject to stockholder approval which was received on June 14, 2017, and was previously amended on June 26, 2018, June 17, 2020, June 10, 2021, June 8, 2022 and June 8, 2023. Our board of directors approved the Amended 2017 Plan on March 27, 2024, subject to stockholder approval at the Annual Meeting.
Prior to the adoption of the 2017 Plan, the Company maintained the Nektar Therapeutics 2012 Plan, as amended (the “2012 Plan”), the Nektar Therapeutics 2008 Equity Incentive Plan, as amended (the “2008 Plan”), the Nektar Therapeutics 2000 Equity Incentive Plan, as amended (the “2000 Plan”), and the Nektar therapeutics 2000 Non-Officer Equity Incentive Plan, as amended (the (“2000 Non-Officer Plan”). The 2000 Non-Officer Plan together with the 2012 Plan, the 2008 Plan and 2000 Plan, are referred to as the “Prior Plans.” Following stockholder approval of the 2017 Plan, no further awards were made under the Prior Plans. As of April 8, 2024, 26,665,748 shares were subject to outstanding stock options and restricted stock units granted under the 2017 Plan and 10,182,402 shares remained available for future decisions regardinggrants under the 2017 Plan. In addition, under the terms of the 2017 Plan, the reserve pool under the 2017 Plan may be increased by shares subject to awards granted under the Prior Plans that were outstanding as of December 31, 2017 in the event that such awards expire, or for any reason are cancelled or terminated, without being exercised. As of April 8, 2024, 0 shares remained subject to outstanding stock options and restricted stock units granted under the Prior Plans. If stockholders approve the amendment to the 2017 Plan, as of April 8, 2024, the number of shares available for future awards under the Amended 2017 Plan will increase by 8,000,000 shares to 18,182,402 shares.
Additional Information on Outstanding Awards and Available Shares under the 2017 Plan and the Prior Plans
The following provides additional information on the total equity compensation awards outstanding and available shares.
| | | As of April 8, 2024 | |
| Shares Outstanding and Available for Grant under the 2017 Plan and the Prior Plans | | | |
| Total shares subject to outstanding stock options | | | 23,000,278 |
| Total shares subject to outstanding deferred restricted stock, restricted stock units, and performance restricted stock units | | | 3,665,470 |
| Weighted-average exercise price of outstanding stock options under all stock incentive plans | | | $8.262 |
| Weighted-average remaining contractual life of outstanding stock options (years) | | | 6.1678 |
Total shares available for grant under all stock incentive plans but not yet granted(1) | | | 10,182,402 |
| (1) | Excludes shares available for purchase under ESPP (774,701 shares as of April 8, 2024) |
Based solely on the closing price of our common stock as reported by the Nasdaq Capital Market on April 8, 2024 and the maximum number of shares that would have been available for awards as of such date, taking into account the proposed increase described herein, the maximum aggregate market value of the common stock that could potentially be issued under the Amended 2017 Plan is $23,818,947.
Given the limited number of shares that currently remain available under the 2017 Plan, our board of directors and management believe it is important that this amendment be approved in order to maintain the Company’s ability to grant stock-based awards to retain employees and continue to provide them with strong incentives to contribute to the Company’s future success. The Company believes that incentives and stock-based awards focus employees on the objective of creating stockholder value and promoting the success of the Company, and that incentive compensation program.plans like the 2017 Plan are an important attraction, retention and motivation tool for participants in the plan.
9
All members of the board of directors and all of the Company’s executive officers will be eligible for awards under the Amended 2017 Plan and thus have a personal interest in the approval of the amendment to the 2017 Plan.
Stockholders are requested in this Proposal 2 to approve the amendment to the 2017 Plan. Approval of the amendment to the 2017 Plan requires the affirmative vote of a majority of the votes cast, by holders of the shares of common stock present during the live webcast or represented by proxy at the Annual Meeting is required (on a non-binding advisory basis) for approval of this proposal. Abstentions are treated as shares representedin person during the live webcast or by proxy, and entitled to vote at the Annual MeetingMeeting. Abstentions and therefore, will have the effect of a vote against this proposal. Brokerbroker non-votes will not have noan effect on the outcome of the vote.vote on this proposal. If stockholders do not approve this amendment, the 2017 Plan will continue in accordance with its current terms.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 4.2.
The essential features of the 2017 Plan, as proposed to be amended, are outlined below:
The principal terms of the Amended 2017 Plan are summarized below. The following summary is qualified in its entirety by the full text of the 2017 Plan and the amendment to the 2017 Plan, which appears as Exhibit A to this proxy statement.
Purpose. The purpose of the Amended 2017 Plan is to promote the success of the Company and the interests of our stockholders by providing an additional means for us to attract, motivate, retain and reward directors, officers, employees and other eligible persons through the grant of awards. Equity-based awards are also intended to further align the interests of award recipients and our stockholders.
Administration. Our board of directors or one or more committees appointed by our board of directors will administer the Amended 2017 Plan. Our board of directors has delegated general administrative authority for the Amended 2017 Plan to the organization and compensation committee of our board of directors. The organization and compensation committee may delegate some or all of its authority with respect to the Amended 2017 Plan to another committee of directors, and certain limited authority to grant awards to employees may be delegated to one or more officers of the Company. (The appropriate acting body, be it the board of directors, a committee within its delegated authority, or an officer within his or her delegated authority, is referred to in this proposal as the “Administrator”).
The Administrator has broad authority under the Amended 2017 Plan with respect to award grants including, without limitation, the authority:
to select participants and determine the type(s) of award(s) that they are to receive;
to determine the number of shares that are to be subject to awards and the terms and conditions of awards, including the price (if any) to be paid for the shares or the award;
to cancel, modify, or waive the Company’s rights with respect to, or modify, discontinue, suspend, or terminate any or all outstanding awards, subject to any required consents;
to accelerate or extend the vesting or exercisability or extend the term of any or all outstanding awards;
subject to the other provisions of the Amended 2017 Plan, to make certain adjustments to an outstanding award and to authorize the termination, conversion, succession or substitution of an award; and
to allow the purchase price of an award or shares of the Company’s common stock to be paid in the form of cash, check, or electronic funds transfer, by the delivery of already-owned shares of the Company’s common stock or by a reduction of the number of shares deliverable pursuant to the award, by services rendered by the recipient of the award, by notice and third party payment or cashless exercise on such terms as the Administrator may authorize, or any other form permitted by law.
No Repricing. In no case (except due to an adjustment to reflect a stock split or other events referred to under “Adjustments” below, or any repricing that may be approved by stockholders) will the Administrator (1) amend an outstanding stock option or stock appreciation right to reduce the exercise price or base price of the award, (2) cancel, exchange, or surrender an outstanding stock option or stock appreciation right in exchange for cash or other awards for the purpose of repricing the award, or (3) cancel, exchange, or surrender an outstanding stock option or stock appreciation right in exchange for an option or stock appreciation right with an exercise or base price that is less than the exercise or base price of the original award.
Eligibility. Persons eligible to receive awards under the Amended 2017 Plan include officers or employees of the Company or any of its subsidiaries, directors of the Company or any of its subsidiaries, and certain consultants and advisors to the Company or any of its subsidiaries. As of April 8, 2024, approximately 138 employees (including executive officers) and six non-employee directors would be eligible to participate in the 2017 Plan.
Authorized Shares; Limits on Awards. Subject to the adjustment provisions included in the 2017 Plan, the maximum number of shares of the Company’s common stock initially available for issuance pursuant to awards under the 2017 Plan equaled 8,300,000 shares of the Company’s common stock (reduced by the number of shares of common stock subject to awards granted under the 2012 Plan on or after March 31, 2017 and prior to the adoption of the 2017 Plan), which was increased to 19,200,000 by stockholder approval in June 2018, was increased to 29,200,000 by stockholder approval in June 2020, was increased to 34,200,000 by stockholder approval in June 2021, was increased to 39,200,000 by stockholder approval in June 2022, and was increased to 51,200,000 by stockholder approval in June 2023. The proposed amendment to the 2017 Plan would increase the total available shares to 59,200,000. Shares issued in respect of any “full-value award” granted under the Amended 2017 Plan will be counted against the share limit described in the preceding sentence as 1.50 shares for every one share actually issued in connection with the award. For example, if the Company granted 100 restricted stock units under the Amended 2017 Plan, 150 shares would be charged against the share limit with respect to that award. For this purpose, a “full-value award” generally means any award granted under the plan other than a stock option or stock appreciation right.
The following other limits are also contained in the Amended 2017 Plan:
the maximum number of shares that may be delivered pursuant to options qualified as incentive stock options granted under the plan is 59,200,000;
the maximum number of shares subject to options and stock appreciation rights that are granted during any calendar year to any individual under the plan is 3,000,000 shares;
“performance-based awards” under Section 5.2 of the Amended 2017 Plan granted to a participant in any one calendar year will not provide for payment of more than (1) in the case of awards payable only in cash and not related to shares, $5,000,000, and (2) in the case of awards related to shares (and in addition to options and stock appreciation rights which are subject to the limit referred to above), 3,000,000 shares; and
the aggregate value of cash compensation and the grant date fair value (computed in accordance with generally accepted accounting principles) of shares of common stock that may be paid or granted during any calendar year to any non-employee director shall not exceed $1,200,000 for existing non-employee directors and $2,200,000 for new non-employee directors.
Except as described in the next sentence, shares that are subject to or underlie awards which expire or for any reason are cancelled or terminated, are forfeited, fail to vest, or for any other reason are not paid or delivered under the Amended 2017 Plan or the Prior Plans will again be available for subsequent awards under the Amended 2017 Plan (with any such shares subject to full-value awards increasing the Amended 2017 Plan’s share limit based on the full-value award ratio described above or, in the case of an award granted under a Prior Plan, the full-value award ratio set forth in such Prior Plan). Shares that are exchanged by a participant or withheld by the Company to pay the exercise price of an award granted under the Amended 2017 Plan, as well as any shares exchanged or withheld to satisfy the tax withholding obligations related to any award, will not be available for subsequent awards under the Amended 2017 Plan. To the extent that an award granted under the Amended 2017 Plan or a Prior Plan is settled in cash or a form other than shares, the shares that would have been delivered had there been no such cash or other settlement will again be available for subsequent awards under the Amended 2017 Plan (with any such shares subject to full-value awards increasing the Amended 2017 Plan’s share limit based on the full-value award ratio described above or, in the case of an award granted under a Prior Plan, the full-value award ratio set forth in such Prior Plan). In the event that shares are delivered in respect of a dividend equivalent right, the actual number of shares delivered with respect to the award shall be counted against the share limits of the Amended 2017 Plan. (For purposes of clarity, if 1,000 dividend equivalent rights are granted and outstanding when the Company pays a dividend, and 50 shares are delivered in payment of those rights with respect to that dividend, 75 shares (after adjustment for the full-value award share counting ratio described above) shall be counted against the share limits of the plan.) To the extent that shares are
11
delivered pursuant to the exercise of a stock appreciation right or stock option, the number of underlying shares as to which the exercise related shall be counted against the applicable share limits, as opposed to only counting the shares issued. (For purposes of clarity, if a stock appreciation right relates to 100,000 shares and is exercised at a time when the payment due to the participant is 15,000 shares, 100,000 shares shall be charged against the applicable share limits with respect to such exercise.) In addition, the Amended 2017 Plan generally provides that shares issued in connection with awards that are granted by or become obligations of the Company through the assumption of awards (or in substitution for awards) in connection with an acquisition of another company will not count against the shares available for issuance under the Amended 2017 Plan. The Company may not increase the applicable share limits of the Amended 2017 Plan by repurchasing shares of common stock on the market (by using cash received through the exercise of stock options or otherwise).
Types of Awards. The Amended 2017 Plan authorizes stock options, stock appreciation rights, stock bonuses, restricted stock, performance stock, stock units, phantom stock or similar rights to purchase or acquire shares, whether at a fixed or variable price or ratio related to the common stock, upon the passage of time, the occurrence of one or more events or the satisfaction of performance criteria or other conditions, awards of any similar securities with a value derived from the value of or related to the common stock and/or returns thereon, or cash awards. The Amended 2017 Plan retains flexibility to offer competitive incentives and to tailor benefits to specific needs and circumstances. Awards granted under the Amended 2017 Plan will be subject to such terms and conditions as established by the Administrator and set forth in the underlying award agreement, including terms relating to the treatment of an award upon a termination of employment. Any award may be paid or settled in cash.
A stock option is the right to purchase shares of the Company’s common stock at a future date at a specified price per share (the “exercise price”). The per share exercise price of an option may not be less than the fair market value of a share of the Company’s common stock on the date of grant. The maximum term of an option is eight years from the date of grant. An option may either be an incentive stock option or a nonqualified stock option. Incentive stock option benefits are taxed differently from nonqualified stock options, as described under “Federal Income Tax Consequences of Awards Under the Amended 2017 Plan” below. Incentive stock options are also subject to more restrictive terms and are limited in amount by the U.S. Internal Revenue Code of 1986, as amended (the “Code”) and the Amended 2017 Plan. Incentive stock options may only be granted to employees of the Company or a subsidiary.
A stock appreciation right is the right to receive payment of an amount equal to the excess of the fair market value of share of the Company’s common stock on the date of exercise of the stock appreciation right over the base price of the stock appreciation right. The base price will be established by the Administrator at the time of grant of the stock appreciation right and may not be less than the fair market value of a share of the Company’s common stock on the date of grant. Stock appreciation rights may be granted in connection with other awards or independently. The maximum term of a stock appreciation right is eight years from the date of grant.
Performance-Based Awards. The Administrator may grant performance-based awards under the Amended 2017 Plan. Performance-based awards are in addition to any of the other types of awards that may be granted under the Amended 2017 Plan. Performance-based awards may be in the form of restricted stock, performance stock, stock units, other rights, or cash bonus opportunities.
The vesting or payment of performance-based awards may depend on the absolute or relative performance of the Company on a consolidated, subsidiary, segment, division, or business unit basis. The Administrator will establish the criterion or criteria and target(s) on which performance will be measured. The criteria that the Administrator may use for this purpose may include, without limitation, any one or more of the following: earnings per share; cash flow (which means cash and cash equivalents derived from either net cash flow from operations or net cash flow from operations, financing and investing activities); working capital; stock price; total stockholder return; revenue; gross profit; operating income; net earnings (before or after interest, taxes, depreciation and/or amortization); gross margin; operating margin; net margin; return on equity or on assets or on net investment; cost containment or reduction; regulatory submissions or approvals; manufacturing production; completion of strategic partnerships; research milestones; any other measure selected by the Administrator or any combination thereof. As applicable, these terms are used as applied under generally accepted accounting principles or in the financial reporting of the Company or of its subsidiaries. The applicable performance goals may be applied on a pre- or post-tax basis and may be adjusted to include or exclude determinable components
12
of any performance goal, including, without limitation, foreign exchange gains and losses, asset write-downs, acquisitions and divestitures, change in fiscal year, unbudgeted capital expenditures, special charges such as restructuring or impairment charges, debt refinancing costs, extraordinary or noncash items, unusual, infrequently occurring, nonrecurring or one-time events affecting the Company or its financial statements or changes in law or accounting principles.
The performance measurement period with respect to an award may range from three months to ten years. Performance-based awards may be paid in stock or in cash (in either case, subject to the limits described under the heading “Authorized Shares; Limits on Awards” above). The Administrator has discretion to determine the performance target or targets and any other restrictions or other limitations of performance-based awards and may reserve discretion to reduce payments below maximum award limits.
Dividend Equivalents; Deferrals. The Administrator may provide for the deferred payment of awards, and may determine the other terms applicable to deferrals. The Administrator may provide that awards under the Amended 2017 Plan (other than options or stock appreciation rights), and/or deferrals, earn dividends or dividend equivalents based on the amount of dividends paid on outstanding shares of common stock, provided that as to any dividends or dividend equivalent rights granted in connection with an award granted under the Amended 2017 Plan that is subject to vesting requirements, no dividends or dividend equivalent payments will be made unless the related vesting conditions of the award are satisfied.
Award Agreements. Each award shall be evidenced by either (1) a written award agreement in a form approved by the Administrator and executed by the Company by an officer duly authorized to act on its behalf, or (2) an electronic notice of award grant in a form approved by the Administrator. The award agreement shall set forth the material terms and conditions of the award as established by the Administrator consistent with the express limitations of the Amended 2017 Plan. Notwithstanding anything in the Amended 2017 Plan to the contrary, the Administrator may approve an award agreement that, upon the termination of a participant’s employment or service, provides that, or may, in its sole discretion based on a review of all relevant facts and circumstances, otherwise take action regarding an award agreement such that (i) any or all outstanding stock options and stock appreciation rights will become exercisable in part or in full, (ii) all or a portion of the restriction or vesting period applicable to any outstanding award will lapse, (iii) all or a portion of the performance measurement period applicable to any outstanding award will lapse and (iv) the performance goals applicable to any outstanding award (if any) will be deemed to be satisfied at the target, maximum or any other interim level.
Assumption and Termination of Awards. Generally, and subject to limited exceptions set forth in the Amended 2017 Plan, upon the occurrence of a “change in control,” as defined in the Amended 2017 Plan, the Administrator may provide for the cash payment in settlement of, or for the termination, assumption, substitution or exchange of any or all outstanding awards granted under the Amended 2017 Plan. To the extent the administrator does not provide for the assumption, substitution or other continuation of the awards, then all awards then-outstanding under the Amended 2017 Plan will become fully vested or paid, as applicable, and will terminate or be terminated in such circumstances, provided that the holder of a stock option or stock appreciation right would be given reasonable advance (but no more than ten days’) notice of the impending termination and a reasonable opportunity to exercise his or her vested stock option or stock appreciation right (after giving effect to any accelerated vesting required in the circumstances) in accordance with their terms before the termination of such awards. The Administrator also has the discretion to establish other change in control provisions with respect to awards granted under the Amended 2017 Plan. For example, the Administrator could provide for the acceleration of vesting or payment of an award in connection with a change in control and provide that any such acceleration shall be automatic upon the occurrence of any such event, including a termination of employment within a limited period of time following a corporate transaction.
Transfer Restrictions. Subject to certain exceptions contained in the Amended 2017 Plan, awards under the Amended 2017 Plan generally are not transferable by the recipient other than by will or the laws of descent and distribution and are generally exercisable, during the recipient’s lifetime, only by the recipient. Any amounts payable or shares issuable pursuant to an award generally will be paid only to the recipient or the recipient’s beneficiary or representative. The Administrator has discretion, however, to establish written conditions and procedures for the transfer of awards to other persons or entities, provided that such transfers comply with
13
applicable federal and state securities laws and are not made for value (other than nominal consideration, settlement of marital property rights, or for interests in an entity in which more than 50% of the voting securities are held by the award recipient or by the recipient’s family members).
Adjustments. As is customary in incentive plans of this nature, each share limit and the number and kind of shares available under the Amended 2017 Plan and any outstanding awards, as well as the exercise or purchase prices of awards, and performance targets under certain types of performance-based awards, are subject to adjustment in the event of certain reorganizations, mergers, combinations, recapitalizations, stock splits, stock dividends, or other similar events that change the number or kind of shares outstanding, and extraordinary dividends or distributions of property to the stockholders.
No Limit on Other Authority. The Amended 2017 Plan does not limit the authority of the board of directors or any committee to grant awards or authorize any other compensation, with or without reference to the Company’s common stock, under any other plan or authority.
Termination of or Changes to the Amended 2017 Plan. The board of directors may amend or terminate the Amended 2017 Plan at any time and in any manner. Stockholder approval for an amendment will be required only to the extent then required by applicable law or any applicable listing agency or required under Sections 422 or 424 of the Code to preserve the intended tax consequences of the plan. Unless terminated earlier by the board of directors, the authority to grant new awards under the Amended 2017 Plan will terminate on March 27, 2027. Outstanding awards, as well as the Administrator’s authority with respect thereto, generally will continue following the expiration or termination of the plan. Generally speaking, outstanding awards may be amended by the Administrator (except for a repricing), but the consent of the award holder is required if the amendment (or any plan amendment) materially and adversely affects the holder.
Clawback Policy. The awards under the Amended 2017 Plan are subject to the terms of the Nektar Therapeutics Compensation Recovery Policy as it may be in effect from time to time.
Federal Income Tax Consequences of Awards under the Amended 2017 Plan
The U.S. federal income tax consequences of the Amended 2017 Plan under current federal law, which is subject to change, are summarized in the following discussion of the general tax principles applicable to the Amended 2017 Plan. This summary is not intended to be exhaustive and, among other considerations, does not describe the deferred compensation provisions of Section 409A of the Code to the extent an award is subject to and does not satisfy those rules, nor does it describe state, local, or international tax consequences.
With respect to nonqualified stock options, the Company is generally entitled to deduct, except to the extent limited by Section 162(m) of the Code, and the participant recognizes taxable income in an amount equal to the difference between the option exercise price and the fair market value of the shares at the time of exercise. With respect to incentive stock options, the Company is generally not entitled to a deduction nor does the participant recognize income at the time of exercise, although if the participant is subject to the U.S. federal alternative minimum tax, the difference between the option exercise price and the fair market value of the shares at the time of exercise is includible for purposes of such alternative minimum tax. If the shares acquired by exercise of an incentive stock option are held for at least two years from the date the option was granted and one year from the date it was exercised, any gain or loss arising from a subsequent disposition of those shares will be taxed as long-term capital gain or loss, and the Company will not be entitled to any deduction. If, however, such shares are disposed of within the above-described period, then in the year of that disposition the participant will recognize compensation taxable as ordinary income equal to the excess of the lesser of (i) the amount realized upon that disposition and (ii) the excess of the fair market value of those shares on the date of exercise over the purchase price, and the Company will be entitled to a corresponding deduction, except to the extent limited by Section 162(m) of the Code.
The current federal income tax consequences of other awards authorized under the Amended 2017 Plan generally follow certain basic patterns: nontransferable restricted stock subject to a substantial risk of forfeiture results in income recognition equal to the excess of the fair market value over the price paid (if any) only at the time the restrictions constituting a substantial risk of forfeiture lapse (unless the recipient elects to accelerate recognition as of the date of grant); bonuses, restricted stock units, stock appreciation rights, cash and stock-based performance awards, dividend equivalents, stock units, and other types of awards are generally subject to tax at the time of payment; and compensation otherwise effectively deferred is taxed when paid. In
14
each of the foregoing cases, the Company will generally have a corresponding deduction at the time the participant recognizes income, except to the extent limited by Section 162(m) of the Code.
If an award is accelerated under the Amended 2017 Plan in connection with a “change in control” (as defined in the Amended 2017 Plan), the Company may not be permitted to deduct the portion of the compensation attributable to the acceleration (“parachute payments”) if it exceeds certain threshold limits under Section 280G of the Code (and certain related excise taxes may be triggered). Furthermore, Section 162(m) of the Code limits to $1 million the amount that a publicly held corporation is allowed each year to deduct for compensation paid to the corporation’s “covered employees.” “Covered employees” include the corporation’s chief executive officer, chief financial officer and three next most highly compensated executive officers. If an individual is determined to be a covered employee for any year beginning after December 31, 2017, then that individual will continue to be a covered employee for future years, regardless of changes in the individual’s compensation or position. Beginning on or after January 1, 2027, the American Rescue Plan Act of 2021 (the “ARPA”) expands the applicability of Section 162(m) of the Code to also include the next five highest paid corporate officers so that the total number of covered employees subject to the $1 million deduction limitation will at least be 10.
New Plan Benefits
The Company has not approved any awards that are conditioned upon stockholder approval of the amendment to the 2017 Plan. The Administrator has the discretion to grant awards under the Amended 2017 Plan and, therefore, it is not possible as of the date of this proxy statement to determine future awards that will be received by the Company’s named executive officers or others under the Amended 2017 Plan. Accordingly, in lieu of providing information regarding benefits that will be received under the Amended 2017 Plan, the following table provides information concerning the benefits that were received by the following person and groups during 2023: each named executive officer; all named executive officers, as a group; all current directors who are not named executive officers, as a group; and all current employees who are not named executive officers, as a group.
| | | Stock Options | | | Restricted Stock Units | |||||||
| Name and Position | | | Number of Shares (#) | | | Average Exercise Price ($) | | | Number of Units (#) | | | Dollar Value ($)(1) |
Howard W. Robin President and Chief Executive Officer | | | 2,600,000 | | | 0.50 | | | — | | | — |
Sandra Gardiner(2) Interim Chief Financial Officer | | | — | | | — | | | — | | | — |
Mark A. Wilson Chief Legal Officer | | | 650,000 | | | 0.50 | | | — | | | — |
Jonathan Zalevsky, Ph.D. Chief Research and Development Officer | | | 500,000 | | | 0.50 | | | — | | | — |
| Named Executive Officer Group (4 persons) | | | 3,750,000 | | | 0.50(3) | | | — | | | — |
| Non-Executive Director Group (6 persons other than Mr. Robin) | | | 510,000 | | | 0.68(3) | | | — | | | — |
| Employee Group (other than named executive officers) (approximately 133 persons)* | | | 7,577,650 | | | 0.51(3) | | | 126,000 | | | 139,014(4) |
| * | As of April 8, 2024 |
| (1) | The valuation of stock awards is based on the grant date fair value computed in accordance with FASB ASC Topic 718. For a discussion of the assumptions used in calculating these values, see Note 10 to our consolidated financial statements in our annual report on Form 10-K for the fiscal year ended December 31, 2023. |
| (2) | Ms. Gardiner was appointed our Interim Chief Financial Officer as of April 17, 2023. Ms. Gardiner is a partner at FLG Partners, LLC and provides services as Interim Chief Financial Officer as an outside consultant pursuant to a consulting agreement between the Company and FLG Partners, LLC. Ms. Gardiner did not receive any equity grant award in 2023. |
| (3) | Represents the weighted average exercise price for the group. |
| (4) | Represents the aggregate grant date fair value for the group. |
15
Equity Compensation Plan Information
The following table presents aggregate summary information as of December 31, 2023, regarding the common stock that may be issued upon the exercise of options and rights under all of our existing equity compensation plans:
| Plan Category | | | Number of Securities to be Issued Upon Exercise of Outstanding Options & Vesting of RSUs (a) | | | Weighted-Average Exercise Price of Outstanding Options (b) | | | Number of Securities Remaining Available for Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column(a)) (c) |
| Equity compensation plans approved by security holders | | | 27,246,000 | | | $8.26 | | | 10,101,000 |
| Equity compensation plans not approved by security holders | | | 0 | | | $0 | | | 0 |
| Total | | | 27,246,000 | | | $8.26 | | | 10,101,000 |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 2 FOR APPROVAL OF THE AMENDMENT TO OUR AMENDED AND RESTATED 2017 PERFORMANCE INCENTIVE
PLAN AS DESCRIBED ABOVE AND SET FORTH IN EXHIBIT A HERETO.
16
The boardAudit Committee of directors values constructive dialogue on executive compensation and other important governance topics with our stockholders. After careful consideration of the strengths and weaknesses of the various potential say-on-pay vote frequency intervals, the board of directors believeshas selected Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2024, and has further directed that management submit the selection of our independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. Ernst & Young LLP has audited our consolidated financial statements since our inception. Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting. They will have an advisory vote every yearopportunity to make a statement if they so desire and will provide an effective way to obtain information on stockholder sentiment about our executive compensation program by allowing adequate time for usbe available to respond to stockholders’ feedback and engage with stockholders to understand and respondappropriate questions.
Neither our bylaws nor other governing documents or law require stockholder ratification of the selection of Ernst & Young LLP as our independent registered public accounting firm. However, the Audit Committee is submitting the selection of Ernst & Young LLP to the vote results.stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Audit Committee will reconsider whether or not to retain Ernst & Young LLP. Even if the selection is ratified, the Audit Committee, in its discretion, may direct the appointment of a different independent registered public accounting firm at any time during the year if the committee determines that such a change would be in our best interests and our stockholders’ best interest.
The choiceaffirmative vote of every year, every two yearsthe holders of a majority of the votes cast during the live webcast or every three years that receivesby proxy at the greatest number of votes from stockholdersAnnual Meeting will be required to ratify the frequencyselection of Ernst & Young LLP for the advisory vote on executive compensation that has been selected by stockholders.our fiscal year ending December 31, 2024. Abstentions and broker non-votes, if any, will have no effect on the outcome of the vote. As an advisory vote, the vote on this Proposal 5 is not binding on us. However, the board of directors values the opinions of our stockholders and will consider the outcome of the vote when setting the frequency of the advisory vote on executive compensation.proposal. No broker non-votes are expected to exist in connection with this proposal.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE FOR AN ANNUAL“ONE YEAR” ADVISORY VOTE ON THE COMPENSATIONIN FAVOR OF THE COMPANY’S NAMED EXECUTIVE OFFICERS AS DISCLOSED IN ITS PROXY STATEMENTS.PROPOSAL 3.
| | | Beneficial Ownership ** | ||||
Beneficial Owner | | | Number of Shares | | | Percent of Total |
Invesco Ltd.(1) | | | 37,327,473 | | | 19.73% |
BlackRock, Inc.(2) | | | 35,928,093 | | | 18.99% |
The Vanguard Group(3) | | | 23,065,775 | | | 12.19% |
PRIMECAP Management Company(4) | | | 15,659,053 | | | 8.27% |
Deep Track Capital, LP(5) | | | 12,302,237 | | | 6.50% |
Jeff Ajer(6) | | | 159,262 | | | * |
Diana Brainard, M.D.(7) | | | 38,080 | | | * |
Robert Chess(8) | | | 274,773 | | | * |
Myriam Curet, M.D.(9) | | | 97,177 | | | * |
Karin Eastham(10) | | | 95,233 | | | * |
R. Scott Greer(11) | | | 410,574 | | | * |
Howard W. Robin(12) | | | 2,282,376 | | | 1.21% |
Roy A. Whitfield(13) | | | 236,050 | | | * |
Jillian B. Thomsen(14) | | | 564,427 | | | * |
Mark A. Wilson(15) | | | 541,243 | | | * |
Jonathan Zalevsky, Ph.D.(16) | | | 1,108,318 | | | * |
All executive officers and directors as a group (11 persons) | | | 5,807,513 | | | 3.07% |
Jeff Ajer
Jeff Ajer, age 61, was appointed to our board of directors in September 2017. Mr. Ajer currently serves as Executive Vice President and Chief Commercial Officer at BioMarin Pharmaceutical Inc. (“BioMarin”), a global biotechnology company that develops and commercializes innovative therapies for people with serious and life-threatening rare disorders. From October 2012 to January 2014, Mr. Ajer served as Senior Vice President and Chief Commercial Officer of BioMarin. From April 2009 to October 2012, Mr. Ajer served as BioMarin’s Vice President, Commercial Operations, The Americas, where he had responsibility for commercial operations throughout the Americas and led product marketing, reimbursement, and sales operations for BioMarin. Prior to joining BioMarin, Mr. Ajer served in various roles at Genzyme Corporation beginning in November 2003, most recently as Vice President, Global Transplant Operations from December 2004 to August 2005. Mr. Ajer’s experience prior to Genzyme Corporation includes roles in sales, marketing and operations at SangStat Medical Corporation and ICN Pharmaceuticals. Mr. Ajer also served on the board of directors of True North Therapeutics until June 2017. Mr. Ajer received both a B.S. in chemistry and an M.B.A. from the University of California, Irvine.
Robert B. Chess
Robert B. Chess, age 67, is the Chairman of our board of directors and has served as a director since May 1992. From March 2006 until January 2007, Mr. Chess served as our Acting President and Chief Executive Officer, and from April 1999 to January 2007, served as Executive Chairman. He also served as our Co-Chief Executive Officer from August 1998 to April 2000, as President from December 1991 to August 1998, and as Chief Executive Officer from May 1992 to August 1998. Mr. Chess was previously the co-founder and President of Penederm, Inc., a publicly-traded dermatological pharmaceutical company that was sold to Mylan Laboratories. He has held management positions at Intel Corporation and Metaphor Computer Systems (now part of IBM), and was a member of the first President Bush’s White House staff as a White House Fellow and Associate Director of the White House Office of Economic and Domestic Policy. Mr. Chess serves on the board of directors and is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is Chairman of two private companies: Bighat Biosciences, which does ML-guided biologics design, and Issio Solutions, which is in the labor productivity software field. From 1997 until his retirement in 2009, Mr. Chess served on the board of directors of the Biotechnology Industry Organization (“BIO”). Mr. Chess served as Chairman of BIO’s Emerging Companies Section and Co-Chairman of BIO’s Intellectual Property Committee. Mr. Chess was the initial Chairman of Bio Ventures for Global Health and served on its board through 2022. Mr. Chess serves on the board of directors and is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is currently a member of the faculty of the Stanford Graduate School of Business, where he teaches courses in the MBA program on the healthcare industry and the business opportunity created by aging demographics and increased longevity. Mr. Chess received his B.S. degree in Engineering with honors from the California Institute of Technology and an M.B.A. from Harvard University.
Roy A. Whitfield
Roy A. Whitfield, age 70, has served as our director since August 2000 and as Lead Independent Director since January 2019. Mr. Whitfield is the former Chairman of the Board and Chief Executive Officer of Incyte Corporation (“Incyte”), a drug discovery and development company he co-founded in 1991. From January 1993 to November 2001, Mr. Whitfield served as its Chief Executive Officer and from November 2001 until June 2003 as its Chairman. He also served as a director of Incyte from 1991 to January 2014. From 1984 to 1989, Mr. Whitfield held senior operating and business development positions with Technicon Instruments Corporation (“Technicon”), a medical instrumentation company, and its predecessor company, Cooper Biomedical, Inc., a biotechnology and medical diagnostics company. Prior to his work at Technicon, Mr. Whitfield spent seven years with the Boston Consulting Group’s international consulting practice. Mr. Whitfield received a B.S. in mathematics from Oxford University and an M.B.A. from Stanford University.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” THE ELECTION OF EACH NAMED
NOMINEE.
8
APPROVAL OF AN AMENDMENT TO OUR AMENDED AND RESTATED 2017 PERFORMANCE INCENTIVE PLAN
At the Annual Meeting, our stockholders will be asked to approve an amendment to the Nektar Therapeutics Amended and Restated 2017 Performance Incentive Plan (the “2017 Plan” and as amended, the “Amended 2017 Plan”) to increase the authorized shares under the Amended 2017 Plan by 8,000,000 shares.The 2017 Plan was originally approved by our board of directors on March 28, 2017, subject to stockholder approval which was received on June 14, 2017, and was previously amended on June 26, 2018, June 17, 2020, June 10, 2021, June 8, 2022 and June 8, 2023. Our board of directors approved the Amended 2017 Plan on March 27, 2024, subject to stockholder approval at the Annual Meeting.
Prior to the adoption of the 2017 Plan, the Company maintained the Nektar Therapeutics 2012 Plan, as amended (the “2012 Plan”), the Nektar Therapeutics 2008 Equity Incentive Plan, as amended (the “2008 Plan”), the Nektar Therapeutics 2000 Equity Incentive Plan, as amended (the “2000 Plan”), and the Nektar therapeutics 2000 Non-Officer Equity Incentive Plan, as amended (the (“2000 Non-Officer Plan”). The 2000 Non-Officer Plan together with the 2012 Plan, the 2008 Plan and 2000 Plan, are referred to as the “Prior Plans.” Following stockholder approval of the 2017 Plan, no further awards were made under the Prior Plans. As of April 8, 2024, 26,665,748 shares were subject to outstanding stock options and restricted stock units granted under the 2017 Plan and 10,182,402 shares remained available for future grants under the 2017 Plan. In addition, under the terms of the 2017 Plan, the reserve pool under the 2017 Plan may be increased by shares subject to awards granted under the Prior Plans that were outstanding as of December 31, 2017 in the event that such awards expire, or for any reason are cancelled or terminated, without being exercised. As of April 8, 2024, 0 shares remained subject to outstanding stock options and restricted stock units granted under the Prior Plans. If stockholders approve the amendment to the 2017 Plan, as of April 8, 2024, the number of shares available for future awards under the Amended 2017 Plan will increase by 8,000,000 shares to 18,182,402 shares.
Additional Information on Outstanding Awards and Available Shares under the 2017 Plan and the Prior Plans
The following provides additional information on the total equity compensation awards outstanding and available shares.
| | | As of April 8, 2024 | |
| Shares Outstanding and Available for Grant under the 2017 Plan and the Prior Plans | | | |
| Total shares subject to outstanding stock options | | | 23,000,278 |
| Total shares subject to outstanding deferred restricted stock, restricted stock units, and performance restricted stock units | | | 3,665,470 |
| Weighted-average exercise price of outstanding stock options under all stock incentive plans | | | $8.262 |
| Weighted-average remaining contractual life of outstanding stock options (years) | | | 6.1678 |
Total shares available for grant under all stock incentive plans but not yet granted(1) | | | 10,182,402 |
| (1) | Excludes shares available for purchase under ESPP (774,701 shares as of April 8, 2024) |
Based solely on the closing price of our common stock as reported by the Nasdaq Capital Market on April 8, 2024 and the maximum number of shares that would have been available for awards as of such date, taking into account the proposed increase described herein, the maximum aggregate market value of the common stock that could potentially be issued under the Amended 2017 Plan is $23,818,947.
Given the limited number of shares that currently remain available under the 2017 Plan, our board of directors and management believe it is important that this amendment be approved in order to maintain the Company’s ability to grant stock-based awards to retain employees and continue to provide them with strong incentives to contribute to the Company’s future success. The Company believes that incentives and stock-based awards focus employees on the objective of creating stockholder value and promoting the success of the Company, and that incentive compensation plans like the 2017 Plan are an important attraction, retention and motivation tool for participants in the plan.
9
All members of the board of directors and all of the Company’s executive officers will be eligible for awards under the Amended 2017 Plan and thus have a personal interest in the approval of the amendment to the 2017 Plan.
Stockholders are requested in this Proposal 2 to approve the amendment to the 2017 Plan. Approval of the amendment to the 2017 Plan requires the affirmative vote of a majority of the votes cast, in person during the live webcast or by proxy, and entitled to vote at the Annual Meeting. Abstentions and broker non-votes will not have an effect on the outcome of the vote on this proposal. If stockholders do not approve this amendment, the 2017 Plan will continue in accordance with its current terms.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 2.
The essential features of the 2017 Plan, as proposed to be amended, are outlined below:
The principal terms of the Amended 2017 Plan are summarized below. The following summary is qualified in its entirety by the full text of the 2017 Plan and the amendment to the 2017 Plan, which appears as Exhibit A to this proxy statement.
Purpose. The purpose of the Amended 2017 Plan is to promote the success of the Company and the interests of our stockholders by providing an additional means for us to attract, motivate, retain and reward directors, officers, employees and other eligible persons through the grant of awards. Equity-based awards are also intended to further align the interests of award recipients and our stockholders.
Administration. Our board of directors or one or more committees appointed by our board of directors will administer the Amended 2017 Plan. Our board of directors has delegated general administrative authority for the Amended 2017 Plan to the organization and compensation committee of our board of directors. The organization and compensation committee may delegate some or all of its authority with respect to the Amended 2017 Plan to another committee of directors, and certain limited authority to grant awards to employees may be delegated to one or more officers of the Company. (The appropriate acting body, be it the board of directors, a committee within its delegated authority, or an officer within his or her delegated authority, is referred to in this proposal as the “Administrator”).
The Administrator has broad authority under the Amended 2017 Plan with respect to award grants including, without limitation, the authority:
to select participants and determine the type(s) of award(s) that they are to receive;
to determine the number of shares that are to be subject to awards and the terms and conditions of awards, including the price (if any) to be paid for the shares or the award;
to cancel, modify, or waive the Company’s rights with respect to, or modify, discontinue, suspend, or terminate any or all outstanding awards, subject to any required consents;
to accelerate or extend the vesting or exercisability or extend the term of any or all outstanding awards;
subject to the other provisions of the Amended 2017 Plan, to make certain adjustments to an outstanding award and to authorize the termination, conversion, succession or substitution of an award; and
to allow the purchase price of an award or shares of the Company’s common stock to be paid in the form of cash, check, or electronic funds transfer, by the delivery of already-owned shares of the Company’s common stock or by a reduction of the number of shares deliverable pursuant to the award, by services rendered by the recipient of the award, by notice and third party payment or cashless exercise on such terms as the Administrator may authorize, or any other form permitted by law.
No Repricing. In no case (except due to an adjustment to reflect a stock split or other events referred to under “Adjustments” below, or any repricing that may be approved by stockholders) will the Administrator (1) amend an outstanding stock option or stock appreciation right to reduce the exercise price or base price of the award, (2) cancel, exchange, or surrender an outstanding stock option or stock appreciation right in exchange for cash or other awards for the purpose of repricing the award, or (3) cancel, exchange, or surrender an outstanding stock option or stock appreciation right in exchange for an option or stock appreciation right with an exercise or base price that is less than the exercise or base price of the original award.
10
Eligibility. Persons eligible to receive awards under the Amended 2017 Plan include officers or employees of the Company or any of its subsidiaries, directors of the Company or any of its subsidiaries, and certain consultants and advisors to the Company or any of its subsidiaries. As of April 8, 2024, approximately 138 employees (including executive officers) and six non-employee directors would be eligible to participate in the 2017 Plan.
Authorized Shares; Limits on Awards. Subject to the adjustment provisions included in the 2017 Plan, the maximum number of shares of the Company’s common stock initially available for issuance pursuant to awards under the 2017 Plan equaled 8,300,000 shares of the Company’s common stock (reduced by the number of shares of common stock subject to awards granted under the 2012 Plan on or after March 31, 2017 and prior to the adoption of the 2017 Plan), which was increased to 19,200,000 by stockholder approval in June 2018, was increased to 29,200,000 by stockholder approval in June 2020, was increased to 34,200,000 by stockholder approval in June 2021, was increased to 39,200,000 by stockholder approval in June 2022, and was increased to 51,200,000 by stockholder approval in June 2023. The proposed amendment to the 2017 Plan would increase the total available shares to 59,200,000. Shares issued in respect of any “full-value award” granted under the Amended 2017 Plan will be counted against the share limit described in the preceding sentence as 1.50 shares for every one share actually issued in connection with the award. For example, if the Company granted 100 restricted stock units under the Amended 2017 Plan, 150 shares would be charged against the share limit with respect to that award. For this purpose, a “full-value award” generally means any award granted under the plan other than a stock option or stock appreciation right.
The following other limits are also contained in the Amended 2017 Plan:
the maximum number of shares that may be delivered pursuant to options qualified as incentive stock options granted under the plan is 59,200,000;
the maximum number of shares subject to options and stock appreciation rights that are granted during any calendar year to any individual under the plan is 3,000,000 shares;
“performance-based awards” under Section 5.2 of the Amended 2017 Plan granted to a participant in any one calendar year will not provide for payment of more than (1) in the case of awards payable only in cash and not related to shares, $5,000,000, and (2) in the case of awards related to shares (and in addition to options and stock appreciation rights which are subject to the limit referred to above), 3,000,000 shares; and
the aggregate value of cash compensation and the grant date fair value (computed in accordance with generally accepted accounting principles) of shares of common stock that may be paid or granted during any calendar year to any non-employee director shall not exceed $1,200,000 for existing non-employee directors and $2,200,000 for new non-employee directors.
Except as described in the next sentence, shares that are subject to or underlie awards which expire or for any reason are cancelled or terminated, are forfeited, fail to vest, or for any other reason are not paid or delivered under the Amended 2017 Plan or the Prior Plans will again be available for subsequent awards under the Amended 2017 Plan (with any such shares subject to full-value awards increasing the Amended 2017 Plan’s share limit based on the full-value award ratio described above or, in the case of an award granted under a Prior Plan, the full-value award ratio set forth in such Prior Plan). Shares that are exchanged by a participant or withheld by the Company to pay the exercise price of an award granted under the Amended 2017 Plan, as well as any shares exchanged or withheld to satisfy the tax withholding obligations related to any award, will not be available for subsequent awards under the Amended 2017 Plan. To the extent that an award granted under the Amended 2017 Plan or a Prior Plan is settled in cash or a form other than shares, the shares that would have been delivered had there been no such cash or other settlement will again be available for subsequent awards under the Amended 2017 Plan (with any such shares subject to full-value awards increasing the Amended 2017 Plan’s share limit based on the full-value award ratio described above or, in the case of an award granted under a Prior Plan, the full-value award ratio set forth in such Prior Plan). In the event that shares are delivered in respect of a dividend equivalent right, the actual number of shares delivered with respect to the award shall be counted against the share limits of the Amended 2017 Plan. (For purposes of clarity, if 1,000 dividend equivalent rights are granted and outstanding when the Company pays a dividend, and 50 shares are delivered in payment of those rights with respect to that dividend, 75 shares (after adjustment for the full-value award share counting ratio described above) shall be counted against the share limits of the plan.) To the extent that shares are
11
delivered pursuant to the exercise of a stock appreciation right or stock option, the number of underlying shares as to which the exercise related shall be counted against the applicable share limits, as opposed to only counting the shares issued. (For purposes of clarity, if a stock appreciation right relates to 100,000 shares and is exercised at a time when the payment due to the participant is 15,000 shares, 100,000 shares shall be charged against the applicable share limits with respect to such exercise.) In addition, the Amended 2017 Plan generally provides that shares issued in connection with awards that are granted by or become obligations of the Company through the assumption of awards (or in substitution for awards) in connection with an acquisition of another company will not count against the shares available for issuance under the Amended 2017 Plan. The Company may not increase the applicable share limits of the Amended 2017 Plan by repurchasing shares of common stock on the market (by using cash received through the exercise of stock options or otherwise).
Types of Awards. The Amended 2017 Plan authorizes stock options, stock appreciation rights, stock bonuses, restricted stock, performance stock, stock units, phantom stock or similar rights to purchase or acquire shares, whether at a fixed or variable price or ratio related to the common stock, upon the passage of time, the occurrence of one or more events or the satisfaction of performance criteria or other conditions, awards of any similar securities with a value derived from the value of or related to the common stock and/or returns thereon, or cash awards. The Amended 2017 Plan retains flexibility to offer competitive incentives and to tailor benefits to specific needs and circumstances. Awards granted under the Amended 2017 Plan will be subject to such terms and conditions as established by the Administrator and set forth in the underlying award agreement, including terms relating to the treatment of an award upon a termination of employment. Any award may be paid or settled in cash.
A stock option is the right to purchase shares of the Company’s common stock at a future date at a specified price per share (the “exercise price”). The per share exercise price of an option may not be less than the fair market value of a share of the Company’s common stock on the date of grant. The maximum term of an option is eight years from the date of grant. An option may either be an incentive stock option or a nonqualified stock option. Incentive stock option benefits are taxed differently from nonqualified stock options, as described under “Federal Income Tax Consequences of Awards Under the Amended 2017 Plan” below. Incentive stock options are also subject to more restrictive terms and are limited in amount by the U.S. Internal Revenue Code of 1986, as amended (the “Code”) and the Amended 2017 Plan. Incentive stock options may only be granted to employees of the Company or a subsidiary.
A stock appreciation right is the right to receive payment of an amount equal to the excess of the fair market value of share of the Company’s common stock on the date of exercise of the stock appreciation right over the base price of the stock appreciation right. The base price will be established by the Administrator at the time of grant of the stock appreciation right and may not be less than the fair market value of a share of the Company’s common stock on the date of grant. Stock appreciation rights may be granted in connection with other awards or independently. The maximum term of a stock appreciation right is eight years from the date of grant.
Performance-Based Awards. The Administrator may grant performance-based awards under the Amended 2017 Plan. Performance-based awards are in addition to any of the other types of awards that may be granted under the Amended 2017 Plan. Performance-based awards may be in the form of restricted stock, performance stock, stock units, other rights, or cash bonus opportunities.
The vesting or payment of performance-based awards may depend on the absolute or relative performance of the Company on a consolidated, subsidiary, segment, division, or business unit basis. The Administrator will establish the criterion or criteria and target(s) on which performance will be measured. The criteria that the Administrator may use for this purpose may include, without limitation, any one or more of the following: earnings per share; cash flow (which means cash and cash equivalents derived from either net cash flow from operations or net cash flow from operations, financing and investing activities); working capital; stock price; total stockholder return; revenue; gross profit; operating income; net earnings (before or after interest, taxes, depreciation and/or amortization); gross margin; operating margin; net margin; return on equity or on assets or on net investment; cost containment or reduction; regulatory submissions or approvals; manufacturing production; completion of strategic partnerships; research milestones; any other measure selected by the Administrator or any combination thereof. As applicable, these terms are used as applied under generally accepted accounting principles or in the financial reporting of the Company or of its subsidiaries. The applicable performance goals may be applied on a pre- or post-tax basis and may be adjusted to include or exclude determinable components
12
of any performance goal, including, without limitation, foreign exchange gains and losses, asset write-downs, acquisitions and divestitures, change in fiscal year, unbudgeted capital expenditures, special charges such as restructuring or impairment charges, debt refinancing costs, extraordinary or noncash items, unusual, infrequently occurring, nonrecurring or one-time events affecting the Company or its financial statements or changes in law or accounting principles.
The performance measurement period with respect to an award may range from three months to ten years. Performance-based awards may be paid in stock or in cash (in either case, subject to the limits described under the heading “Authorized Shares; Limits on Awards” above). The Administrator has discretion to determine the performance target or targets and any other restrictions or other limitations of performance-based awards and may reserve discretion to reduce payments below maximum award limits.
Dividend Equivalents; Deferrals. The Administrator may provide for the deferred payment of awards, and may determine the other terms applicable to deferrals. The Administrator may provide that awards under the Amended 2017 Plan (other than options or stock appreciation rights), and/or deferrals, earn dividends or dividend equivalents based on the amount of dividends paid on outstanding shares of common stock, provided that as to any dividends or dividend equivalent rights granted in connection with an award granted under the Amended 2017 Plan that is subject to vesting requirements, no dividends or dividend equivalent payments will be made unless the related vesting conditions of the award are satisfied.
Award Agreements. Each award shall be evidenced by either (1) a written award agreement in a form approved by the Administrator and executed by the Company by an officer duly authorized to act on its behalf, or (2) an electronic notice of award grant in a form approved by the Administrator. The award agreement shall set forth the material terms and conditions of the award as established by the Administrator consistent with the express limitations of the Amended 2017 Plan. Notwithstanding anything in the Amended 2017 Plan to the contrary, the Administrator may approve an award agreement that, upon the termination of a participant’s employment or service, provides that, or may, in its sole discretion based on a review of all relevant facts and circumstances, otherwise take action regarding an award agreement such that (i) any or all outstanding stock options and stock appreciation rights will become exercisable in part or in full, (ii) all or a portion of the restriction or vesting period applicable to any outstanding award will lapse, (iii) all or a portion of the performance measurement period applicable to any outstanding award will lapse and (iv) the performance goals applicable to any outstanding award (if any) will be deemed to be satisfied at the target, maximum or any other interim level.
Assumption and Termination of Awards. Generally, and subject to limited exceptions set forth in the Amended 2017 Plan, upon the occurrence of a “change in control,” as defined in the Amended 2017 Plan, the Administrator may provide for the cash payment in settlement of, or for the termination, assumption, substitution or exchange of any or all outstanding awards granted under the Amended 2017 Plan. To the extent the administrator does not provide for the assumption, substitution or other continuation of the awards, then all awards then-outstanding under the Amended 2017 Plan will become fully vested or paid, as applicable, and will terminate or be terminated in such circumstances, provided that the holder of a stock option or stock appreciation right would be given reasonable advance (but no more than ten days’) notice of the impending termination and a reasonable opportunity to exercise his or her vested stock option or stock appreciation right (after giving effect to any accelerated vesting required in the circumstances) in accordance with their terms before the termination of such awards. The Administrator also has the discretion to establish other change in control provisions with respect to awards granted under the Amended 2017 Plan. For example, the Administrator could provide for the acceleration of vesting or payment of an award in connection with a change in control and provide that any such acceleration shall be automatic upon the occurrence of any such event, including a termination of employment within a limited period of time following a corporate transaction.
Transfer Restrictions. Subject to certain exceptions contained in the Amended 2017 Plan, awards under the Amended 2017 Plan generally are not transferable by the recipient other than by will or the laws of descent and distribution and are generally exercisable, during the recipient’s lifetime, only by the recipient. Any amounts payable or shares issuable pursuant to an award generally will be paid only to the recipient or the recipient’s beneficiary or representative. The Administrator has discretion, however, to establish written conditions and procedures for the transfer of awards to other persons or entities, provided that such transfers comply with
13
applicable federal and state securities laws and are not made for value (other than nominal consideration, settlement of marital property rights, or for interests in an entity in which more than 50% of the voting securities are held by the award recipient or by the recipient’s family members).
Adjustments. As is customary in incentive plans of this nature, each share limit and the number and kind of shares available under the Amended 2017 Plan and any outstanding awards, as well as the exercise or purchase prices of awards, and performance targets under certain types of performance-based awards, are subject to adjustment in the event of certain reorganizations, mergers, combinations, recapitalizations, stock splits, stock dividends, or other similar events that change the number or kind of shares outstanding, and extraordinary dividends or distributions of property to the stockholders.
No Limit on Other Authority. The Amended 2017 Plan does not limit the authority of the board of directors or any committee to grant awards or authorize any other compensation, with or without reference to the Company’s common stock, under any other plan or authority.
Termination of or Changes to the Amended 2017 Plan. The board of directors may amend or terminate the Amended 2017 Plan at any time and in any manner. Stockholder approval for an amendment will be required only to the extent then required by applicable law or any applicable listing agency or required under Sections 422 or 424 of the Code to preserve the intended tax consequences of the plan. Unless terminated earlier by the board of directors, the authority to grant new awards under the Amended 2017 Plan will terminate on March 27, 2027. Outstanding awards, as well as the Administrator’s authority with respect thereto, generally will continue following the expiration or termination of the plan. Generally speaking, outstanding awards may be amended by the Administrator (except for a repricing), but the consent of the award holder is required if the amendment (or any plan amendment) materially and adversely affects the holder.
Clawback Policy. The awards under the Amended 2017 Plan are subject to the terms of the Nektar Therapeutics Compensation Recovery Policy as it may be in effect from time to time.
Federal Income Tax Consequences of Awards under the Amended 2017 Plan
The U.S. federal income tax consequences of the Amended 2017 Plan under current federal law, which is subject to change, are summarized in the following discussion of the general tax principles applicable to the Amended 2017 Plan. This summary is not intended to be exhaustive and, among other considerations, does not describe the deferred compensation provisions of Section 409A of the Code to the extent an award is subject to and does not satisfy those rules, nor does it describe state, local, or international tax consequences.
With respect to nonqualified stock options, the Company is generally entitled to deduct, except to the extent limited by Section 162(m) of the Code, and the participant recognizes taxable income in an amount equal to the difference between the option exercise price and the fair market value of the shares at the time of exercise. With respect to incentive stock options, the Company is generally not entitled to a deduction nor does the participant recognize income at the time of exercise, although if the participant is subject to the U.S. federal alternative minimum tax, the difference between the option exercise price and the fair market value of the shares at the time of exercise is includible for purposes of such alternative minimum tax. If the shares acquired by exercise of an incentive stock option are held for at least two years from the date the option was granted and one year from the date it was exercised, any gain or loss arising from a subsequent disposition of those shares will be taxed as long-term capital gain or loss, and the Company will not be entitled to any deduction. If, however, such shares are disposed of within the above-described period, then in the year of that disposition the participant will recognize compensation taxable as ordinary income equal to the excess of the lesser of (i) the amount realized upon that disposition and (ii) the excess of the fair market value of those shares on the date of exercise over the purchase price, and the Company will be entitled to a corresponding deduction, except to the extent limited by Section 162(m) of the Code.
The current federal income tax consequences of other awards authorized under the Amended 2017 Plan generally follow certain basic patterns: nontransferable restricted stock subject to a substantial risk of forfeiture results in income recognition equal to the excess of the fair market value over the price paid (if any) only at the time the restrictions constituting a substantial risk of forfeiture lapse (unless the recipient elects to accelerate recognition as of the date of grant); bonuses, restricted stock units, stock appreciation rights, cash and stock-based performance awards, dividend equivalents, stock units, and other types of awards are generally subject to tax at the time of payment; and compensation otherwise effectively deferred is taxed when paid. In
14
each of the foregoing cases, the Company will generally have a corresponding deduction at the time the participant recognizes income, except to the extent limited by Section 162(m) of the Code.
If an award is accelerated under the Amended 2017 Plan in connection with a “change in control” (as defined in the Amended 2017 Plan), the Company may not be permitted to deduct the portion of the compensation attributable to the acceleration (“parachute payments”) if it exceeds certain threshold limits under Section 280G of the Code (and certain related excise taxes may be triggered). Furthermore, Section 162(m) of the Code limits to $1 million the amount that a publicly held corporation is allowed each year to deduct for compensation paid to the corporation’s “covered employees.” “Covered employees” include the corporation’s chief executive officer, chief financial officer and three next most highly compensated executive officers. If an individual is determined to be a covered employee for any year beginning after December 31, 2017, then that individual will continue to be a covered employee for future years, regardless of changes in the individual’s compensation or position. Beginning on or after January 1, 2027, the American Rescue Plan Act of 2021 (the “ARPA”) expands the applicability of Section 162(m) of the Code to also include the next five highest paid corporate officers so that the total number of covered employees subject to the $1 million deduction limitation will at least be 10.
New Plan Benefits
The Company has not approved any awards that are conditioned upon stockholder approval of the amendment to the 2017 Plan. The Administrator has the discretion to grant awards under the Amended 2017 Plan and, therefore, it is not possible as of the date of this proxy statement to determine future awards that will be received by the Company’s named executive officers or others under the Amended 2017 Plan. Accordingly, in lieu of providing information regarding benefits that will be received under the Amended 2017 Plan, the following table provides information concerning the benefits that were received by the following person and groups during 2023: each named executive officer; all named executive officers, as a group; all current directors who are not named executive officers, as a group; and all current employees who are not named executive officers, as a group.
| | | Stock Options | | | Restricted Stock Units | |||||||
| Name and Position | | | Number of Shares (#) | | | Average Exercise Price ($) | | | Number of Units (#) | | | Dollar Value ($)(1) |
Howard W. Robin President and Chief Executive Officer | | | 2,600,000 | | | 0.50 | | | — | | | — |
Sandra Gardiner(2) Interim Chief Financial Officer | | | — | | | — | | | — | | | — |
Mark A. Wilson Chief Legal Officer | | | 650,000 | | | 0.50 | | | — | | | — |
Jonathan Zalevsky, Ph.D. Chief Research and Development Officer | | | 500,000 | | | 0.50 | | | — | | | — |
| Named Executive Officer Group (4 persons) | | | 3,750,000 | | | 0.50(3) | | | — | | | — |
| Non-Executive Director Group (6 persons other than Mr. Robin) | | | 510,000 | | | 0.68(3) | | | — | | | — |
| Employee Group (other than named executive officers) (approximately 133 persons)* | | | 7,577,650 | | | 0.51(3) | | | 126,000 | | | 139,014(4) |
| * | As of April 8, 2024 |
| (1) | The valuation of stock awards is based on the grant date fair value computed in accordance with FASB ASC Topic 718. For a discussion of the assumptions used in calculating these values, see Note 10 to our consolidated financial statements in our annual report on Form 10-K for the fiscal year ended December 31, 2023. |
| (2) | Ms. Gardiner was appointed our Interim Chief Financial Officer as of April 17, 2023. Ms. Gardiner is a partner at FLG Partners, LLC and provides services as Interim Chief Financial Officer as an outside consultant pursuant to a consulting agreement between the Company and FLG Partners, LLC. Ms. Gardiner did not receive any equity grant award in 2023. |
| (3) | Represents the weighted average exercise price for the group. |
| (4) | Represents the aggregate grant date fair value for the group. |
15
Equity Compensation Plan Information
The following table presents aggregate summary information as of December 31, 2023, regarding the common stock that may be issued upon the exercise of options and rights under all of our existing equity compensation plans:
| Plan Category | | | Number of Securities to be Issued Upon Exercise of Outstanding Options & Vesting of RSUs (a) | | | Weighted-Average Exercise Price of Outstanding Options (b) | | | Number of Securities Remaining Available for Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column(a)) (c) |
| Equity compensation plans approved by security holders | | | 27,246,000 | | | $8.26 | | | 10,101,000 |
| Equity compensation plans not approved by security holders | | | 0 | | | $0 | | | 0 |
| Total | | | 27,246,000 | | | $8.26 | | | 10,101,000 |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 2 FOR APPROVAL OF THE AMENDMENT TO OUR AMENDED AND RESTATED 2017 PERFORMANCE INCENTIVE
PLAN AS DESCRIBED ABOVE AND SET FORTH IN EXHIBIT A HERETO.
16
RATIFICATION OF SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Audit Committee of the board of directors has selected Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2024, and has further directed that management submit the selection of our independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. Ernst & Young LLP has audited our consolidated financial statements since our inception. Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither our bylaws nor other governing documents or law require stockholder ratification of the selection of Ernst & Young LLP as our independent registered public accounting firm. However, the Audit Committee is submitting the selection of Ernst & Young LLP to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Audit Committee will reconsider whether or not to retain Ernst & Young LLP. Even if the selection is ratified, the Audit Committee, in its discretion, may direct the appointment of a different independent registered public accounting firm at any time during the year if the committee determines that such a change would be in our best interests and our stockholders’ best interest.
The affirmative vote of the holders of a majority of the votes cast during the live webcast or by proxy at the Annual Meeting will be required to ratify the selection of Ernst & Young LLP for our fiscal year ending December 31, 2024. Abstentions and broker non-votes, if any, will have no effect on the outcome of the vote on this proposal. No broker non-votes are expected to exist in connection with this proposal.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 3.
17
ADVISORY VOTE ON EXECUTIVE COMPENSATION
The board of directors is committed to excellence in governance and is aware of the significant interest in executive compensation matters by investors and the general public.
We have designed our executive compensation program to attract, motivate, reward and retain the senior management talent required to achieve our corporate objectives and increase stockholder value. We believe that our compensation policies and procedures are centered on pay-for-performance principles and are strongly aligned with the long-term interests of our stockholders.
We urge you to carefully review the Compensation Discussion and Analysis section of this proxy statement for details on our executive compensation, including our compensation philosophy and objectives and the 2023 compensation of the named executive officers (“NEOs”).
We are presenting this proposal, which gives you as a stockholder the opportunity to endorse or not endorse our compensation program for the NEOs by voting for or against the following resolution (a “say-on-pay” vote), as required pursuant to Section 14A of the Exchange Act:
“RESOLVED, that the compensation paid to the Company’s NEOs, as disclosed pursuant to Item 402 of Regulation S-K promulgated by the SEC, including the Compensation Discussion and Analysis, compensation tables and related narrative discussion contained in the proxy statement for the Company’s 2024 Annual Meeting is hereby APPROVED.”
While the vote on the resolution is advisory in nature and therefore will not bind us to take any particular action, our board of directors and our Organization and Compensation Committee intend to carefully consider the stockholder vote resulting from the proposal in making future decisions regarding our compensation program.
The affirmative vote of a majority of the votes cast by holders of the shares of common stock present during the live webcast or represented by proxy at the Annual Meeting is required (on a non-binding advisory basis) for approval of this proposal. Abstentions and broker non-votes will have no effect on the outcome of the vote on this proposal.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 4.
18
The following table sets forth certain information regarding the ownership of our common stock as of April 8, 2024, by: (i) each director and nominee for director; (ii) each of our NEOs; (iii) all of our executive officers and directors as a group; and (iv) all those known by us to be beneficial owners of more than five percent of our common stock.
Unless otherwise noted below, the address of each beneficial owner listed in the table is c/o Nektar Therapeutics, 455 Mission Bay Boulevard South, San Francisco, California 94158.
| | | Beneficial Ownership** | ||||
| Beneficial Owner | | | Number of Shares | | | Percent of Total |
TCG Crossover GP II, LLC(1) | | | 20,046,350 | | | 10.92% |
Deep Track Capital, LP(2) | | | 18,400,000 | | | 10.02% |
The Vanguard Group(3) | | | 12,042,873 | | | 6.56 |
Jeff Ajer(4) | | | 228,567 | | | * |
Diana Brainard, M.D.(5) | | | 130,103 | | | * |
Robert Chess(6) | | | 455,437 | | | * |
Myriam Curet, M.D.(7) | | | 166,482 | | | * |
R. Scott Greer(8) | | | 484,238 | | | * |
Howard W. Robin(9) | | | 2,839,358 | | | 1.55% |
Roy A. Whitfield(10) | | | 468,414 | | | * |
| Sandra Gardiner | | | — | | | — |
Mark A. Wilson(11) | | | 759,520 | | | * |
Jonathan Zalevsky, Ph.D.(12) | | | 1,279,828 | | | * |
| All executive officers and directors as a group (10 persons) | | | 6,811,947 | | | 3.71% |
| * | Denotes ownership percentage less than 1%. |
| ** | This table is based upon information supplied by officers, directors and principal stockholders and Schedules 13G filed with the SEC. Unless otherwise indicated in the footnotes to this table, and subject to community property laws where applicable, we believe that each of the stockholders named in the table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on 183,624,232 shares outstanding on April 8, 2024, adjusted as required by rules promulgated by the SEC. |
| 1. | Based solely on the Schedule 13G filed with the SEC on March 15, 2024 by TCG Crossover GP II, LLC, TCG Crossover Fund II, L.P, and Mr. Chen Yu. Each of TCG Crossover GP II, LLC, TCG Crossover Fund II, L.P, and Mr. Chen Yu have shared voting and dispositive power over 20,046,350 shares of our common stock (consists of (i) 3,000,000 shares of our common stock and (ii) 17,046,350 shares of our common stock, issuable upon exercise of certain Pre-Funded Warrants (as defined and described in our Current Report on Form 8-K filed with the SEC on March 4, 2024) and excludes 7,953,650 shares of our common stock issuable upon exercise of certain Pre-Funded Warrants because the Pre-Funded Warrants may not be exercised to the extent that doing so would result in the holder of the Pre-Funded Warrants (together with the holder’s affiliates and any other persons acting as a group together with the holder or any of the holder’s affiliates) beneficially owning more than 9.99% of the shares of our common stock then outstanding immediately after giving effect to such exercise). The principal business address of each of TCG Crossover GP II, LLC, TCG Crossover Fund II, L.P, and Mr. Chen Yu is 705 High St., Palo Alto, CA 94301. |
| 2. | Based solely on the Schedule 13G/A (Amendment No. 1) filed with the SEC on February 14, 2024 by Deep Track Capital, LP, Deep Track Biotechnology Master Fund, Ltd. and Mr. David Kroin. Each of Deep Track Capital, LP, Deep Track Biotechnology Master Fund, Ltd. and Mr. David Kroin have shared voting and dispositive power over 18,400,000 shares of our common stock. The principal business address of Deep Track Capital, LP and Mr. David Kroin is 200 Greenwich Ave, 3rd Floor, Greenwich, CT 06830. The principal business address of Deep Track Biotechnology Master Fund, Ltd. is c/o Walkers Corporate Limited, 190 Elgin Ave, George Town, KY1-9001, Cayman Islands. |
| 3. | Based solely on the Schedule 13G/A (Amendment No. 13) filed with the SEC on February 13, 2024 by The Vanguard Group Inc., a registered investment adviser in accordance with Rule 240.13d-1(b)(1)(ii)(E). The Vanguard Group has sole dispositive power over 11,974,323 shares of our common stock and shared dispositive power over 68,550 shares of our common stock. The address of The Vanguard Group is 100 Vanguard Blvd., Malvern, PA 19355. |
| 4. | Includes 194,414 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024. |
| 5. | Includes 107,664 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024. |
| 6. | Includes 180,664 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024. |
| 7. | Includes 139,064 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024. |
| 8. | Includes 180,664 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024. |
| 9. | Includes (i) 2,191,745 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024, (ii) 44,723 shares from RSU awards that are scheduled to vest and be released within 60 days of April 8, 2024 and (iii) 410 shares owned by Mr. Robin’s spouse. |
19
| 10. | Includes (i) 180,664 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024 and (ii) 71,500 shares held in trusts for Mr. Whitfield’s children under which Mr. Whitfield is the sole trustee. |
| 11. | Includes (i) 598,287 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024, (ii) 16,978 shares from RSU awards that are scheduled to vest and be released within 60 days of April 8, 2024 and (iii) 6,107 shares issued pursuant to our Amended and Restated Employee Stock Purchase Plan. |
| 12. | Includes (i) 1,709,878 shares issuable upon exercise of stock options exercisable within 60 days of April 8, 2024 and (ii) 15,663 shares from RSU awards that are scheduled to vest and be released within 60 days of April 8, 2024. |
SECTION 16(A) REPORTS
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who beneficially own more than ten percent of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock and other equity securities. Officers, directors and greater than ten percent beneficial owners are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on our review of Forms 3, 4 and 5, and any amendments thereto, furnished to us or written representations that no Form 5 was required, we believe that during the fiscal year ended December 31, 2023, all filing requirements applicable to our executive officers and directors under the Exchange Act were met in a timely manner.
20
We review all relationships and transactions between us and (i) any of our directors or executive officers, (ii) any nominee for election as a director, (iii) any security holder who is known to us to own beneficially or of record more than five percent of our common stock or (iv) any member of the immediate family of any of the foregoing. Our legal staff is primarily responsible for the development and implementation of processes and controls to obtain information with respect to related person transactions and for then determining, based on the facts and circumstances, whether the Company or a related person has a direct or indirect material interest in the transaction. In addition, the Audit Committee reviews and approves or ratifies any related person transaction that is required to be disclosed. In the course of its review and approval or ratification of a disclosable related person transaction, the committee considers:
the nature of the related person’s interest in the transaction;
the material terms of the transaction, including, without limitation, the dollar amount and type of transaction;
the importance of the transaction to the related person;
whether the transaction would impair the judgment of a director or executive officer to act in the best interest of the Company; and
any other matters the committee deems appropriate.
Any member of the Audit Committee who is a related person with respect to a transaction under review may not participate in the deliberations or vote respecting approval or ratification of the transaction; however, such director may be counted in determining the presence of a quorum at a meeting where the Audit Committee reviews the transaction.
As required under SEC rules, related party transactions that are determined to be directly or indirectly material to us or the related party are disclosed in our proxy statement. Historically, we have not entered into transactions with related parties. On April 7, 2023, we entered into a consulting agreement with FLG Partners, LLC (“FLG Partners”), pursuant to which Sandra Gardiner, a partner at FLG Partners, provides consulting services to us and serves as our Interim Chief Financial Officer. In 2023, we paid FLG Partners $376,300 for the consulting services of Ms. Gardiner and $3,476.68 for reimbursement of out-of-pocket travel expenses.
During the 2023 fiscal year, there were no other relationships or transactions between us and any related party for which disclosure is required under the rules of the SEC.
21
The following is a brief biography of each current director, including each nominee for reelection at the Annual Meeting to a new term of office and each director whose current term of office continues through the Annual Meeting.
Diana M. Brainard, M.D.
Diana M. Brainard, M.D., age 53, was appointed to our board of directors in November 2021. Dr. Brainard currently serves as the Chief Executive Officer and a member of the board of directors of AlloVir, Inc., a late clinical-stage cell therapy company. Prior to joining AlloVir, Inc., Dr. Brainard served as Senior Vice President and Virology Therapeutic Area Head at Gilead Sciences, Inc. from 2018 to April 2021. From 2015 to 2018, Dr. Brainard served as Vice President of Clinical Research, Liver Diseases at Gilead Sciences, Inc. Dr. Brainard obtained her B.A. degree from Brown University and her M.D. from Tulane University School of Medicine.
R. Scott Greer
R. Scott Greer, age 65,has served as our director since February 2010. Mr. Greer currently serves as Managing Director of Numenor Ventures, LLC, a venture capital firm. In 1996, Mr. Greer co-founded Abgenix, Inc., a company that specialized in the discovery, development and manufacture of human therapeutic antibodies, and from June 1996 through May 2002, he served as its Chief Executive Officer. He previously also served as a director of Abgenix from 1996 and Chairman of the board of directors from 2000 until the acquisition of Abgenix by Amgen, Inc. in April 2006. Prior to Abgenix’s formation, Mr. Greer held senior management positions at Cell Genesys, Inc., a biotechnology company, initially as Chief Financial Officer and Vice President of Corporate Development and later as Senior Vice President of Corporate Development, and various positions at Genetics Institute, Inc., a biotechnology research and development company. He previously served on the board of directors of Inogen, Inc., a medical device company that develops and markets oxygen therapy products from 2015-2021, Sientra, Inc., a medical aesthetics company from 2014-2018,Versartis, Inc., an endocrine focused biopharmaceutical company from 2014-2018, Auspex Pharmaceuticals, a biopharmaceutical company developing drugs for patients with movement disorders and other rare diseases from 2014-2015, StemCells, Inc., a biopharmaceutical company focused on stem cell therapeutics from 2010-2016, Ablexis, an antibody technology company, as its Chairman of the board of directors from 2010-2016, Sirna Therapeutics, Inc., a biotechnology company, from 2003, and as its Chairman of the board of directors from 2005, through the closing of the acquisition of Sirna by Merck & Co., Inc. in December 2006. Mr. Greer previously also served as a member of the board of directors of Illumina, Inc., a provider of integrated systems for the analysis of genetic variation and biological function from 2001-2005 and of the board of directors of CV Therapeutics, Inc., a biotechnology company from 2001-2004. Mr. Greer received a B.A. in Economics from Whitman College and an M.B.A. degree from Harvard University. He also was a certified public accountant.
Myriam J. Curet, M.D.
Myriam Curet, M.D., age 67, was appointed to serve as a member of our board of directors in December 2019. Dr. Curet currently serves as the Executive Vice President and Chief Medical Officer of Intuitive Surgical, Inc. Prior to being promoted as Executive Vice President and Chief Medical Officer in November 2017, Dr. Curet served as the Chief Medical Advisor for Intuitive Surgical from December 2005 to February 2014 and as Intuitive Surgical’s Senior Vice President and Chief Medical Officer from February 2014 to November 2017. Dr. Curet has served on the board of directors of Stereotaxis, Inc. since July 2021 and has served on the board of directors of Inspire Medical Systems, Inc. since December 2023. Dr. Curet also has a faculty position as Professor of Surgery at Stanford University School of Medicine. Since October 2010, she has served as a Consulting Professor of Surgery at Stanford University with a part time clinical appointment at the Palo Alto Veteran’s Administration Medical Center. She was also on the faculty at the University of New Mexico for six years prior to joining Stanford University in 2000. Dr. Curet received her M.D. from Harvard Medical School and completed her general surgery residency program at the University of Chicago and her Surgical Endoscopy fellowship at the University of New Mexico.
22
Howard W. Robin
Howard W. Robin, age 71, has served as our President and Chief Executive Officer since January 2007 and has served as a member of our board of directors since February 2007. Mr. Robin served as Chief Executive Officer, President and a director of Sirna Therapeutics, Inc., a biotechnology company, from July 2001 to November 2006 and from January 2001 to June 2001, served as their Chief Operating Officer, President and as a director. From 1991 to 2001, Mr. Robin was Corporate Vice President and General Manager at Berlex Laboratories, Inc. (“Berlex”), a pharmaceutical products company that is a subsidiary of Schering, AG, and from 1987 to 1991 he served as Vice President of Finance and Business Development and Chief Financial Officer. From 1984 to 1987, Mr. Robin was Director of Business Planning and Development at Berlex. He was a Senior Associate with Arthur Andersen & Co. prior to joining Berlex. He received his B.S. in Accounting and Finance from Fairleigh Dickinson University and serves as a member of its Board of Trustees.
Jeff Ajer
Jeff Ajer, age 61, was appointed to our board of directors in September 2017. Mr. Ajer currently serves as Executive Vice President and Chief Commercial Officer at BioMarin Pharmaceutical Inc. (“BioMarin”), a global biotechnology company that develops and commercializes innovative therapies for people with serious and life-threatening rare disorders. From October 2012 to January 2014, Mr. Ajer served as Senior Vice President and Chief Commercial Officer of BioMarin. From April 2009 to October 2012, Mr. Ajer served as BioMarin’s Vice President, Commercial Operations, The Americas, where he had responsibility for commercial operations throughout the Americas and led product marketing, reimbursement, and sales operations for BioMarin. Prior to joining BioMarin, Mr. Ajer served in various roles at Genzyme Corporation (“Genzyme”) beginning in November 2003, most recently as Vice President, Global Transplant Operations from December 2004 to August 2005. Mr. Ajer’s experience prior to Genzyme includes roles in sales, marketing and operations at SangStat Medical Corporation and ICN Pharmaceuticals. Mr. Ajer also served on the board of directors of True North Therapeutics until June 2017. Mr. Ajer received both a B.S. in chemistry and an M.B.A. from the University of California, Irvine.
Robert B. Chess
Robert B. Chess, age 66,67, is the Chairman of our board of directors and has served as a director since May 1992. From March 2006 until January 2007, Mr. Chess served as our Acting President and Chief Executive Officer, and from April 1999 to January 2007, served as Executive Chairman. He also served as our Co-Chief Executive Officer from August 1998 to April 2000, as President from December 1991 to August 1998, and as Chief Executive Officer from May 1992 to August 1998. Mr. Chess was previously the co-founder and President of Penederm, Inc., a publicly-traded dermatological pharmaceutical company that was sold to Mylan Laboratories. He has held management positions at Intel Corporation and Metaphor Computer Systems (now part of IBM), and was a member of the first President Bush’s White House staff as a White House Fellow and Associate Director of the White House Office of Economic and Domestic Policy. Mr. Chess serves on the board of directors and is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is Chairman of two private companies: Bighat Biosciences, which does ML-guided biologics design, and Issio Solutions, which is in the labor productivity software field. From 1997 until his retirement in 2009, Mr. Chess served on the board of directors of the Biotechnology Industry Organization (“BIO”). Mr. Chess served as Chairman of BIO’s Emerging Companies Section and Co-Chairman of BIO’s Intellectual Property Committee. Mr. Chess was the initial Chairman of Bio Ventures for Global Health and continues to serveserved on its board.Board through 2022. He also serves on the Board of Trustees of the California Institute of Technology where he chairs the Technology Transfer Committee. Mr. Chess serves on the board of directors andcurrently is the lead director of Twist Biosciences, a publicly-traded company in the synthetic biology field. He is currently a member of the faculty of the Stanford Graduate School of Business, where he teaches courses in the MBA program on the healthcare industry and the business opportunity created by aging demographics and increased longevity. Mr. Chess received his B.S. degree in Engineering with honors from the California Institute of Technology and an M.B.A. from Harvard University.
23
Roy A. Whitfield
Roy A. Whitfield, age 69,70, has served as our director since August 2000 and as Lead Independent Director since January 2019. Mr. Whitfield is the former Chairman of the Board and Chief Executive Officer of Incyte Corporation (“Incyte”), a drug discovery and development company he co-founded in 1991. From January 1993 to November 2001, Mr. Whitfield served as its Chief Executive Officer and from November 2001 until June 2003 as its Chairman. He also served as a director of Incyte from 1991 to January 2014. From 1984 to 1989, Mr. Whitfield held senior operating and business development positions with Technicon Instruments Corporation (“Technicon”), a medical instrumentation company, and its predecessor company, Cooper Biomedical, Inc., a biotechnology and medical diagnostics company. Prior to his work at Technicon, Mr. Whitfield spent seven years with the Boston Consulting Group’s international consulting practice. Mr. Whitfield received a B.S. in mathematics from Oxford University and an M.B.A. from Stanford University.
The board of directors met twelve (12)thirteen (13) times during 2022.2023. For the term of service during which he or she was a director in fiscal year 2021,2023, each board member attended 75% or more of the aggregate of the board meetings and key committee meetings. All of our directors on our board attended our 20222023 annual meeting of stockholders.
The board of directors has documented our governance practices in our Corporate Governance Policy Statement to assure that the board will have the necessary authority and practices in place to review and evaluate our business operations as needed and to make decisions that are independent of our management. The guidelines are also intended to align the interests of directors and management with those of our stockholders. The Corporate Governance Policy Statement sets forth certain practices the board will follow with respect to board composition, board committees, board nomination, director qualifications and evaluation of the board and committees. The Corporate Governance Policy Statement also provides that the board of directors will include qualified candidates when filling positions for Chief Executive Officer vacancies and board membership from a variety of backgrounds and experiences, including candidates of gender, age and racial/ethnic diversity. In any retained search for Chief Executive Officer and board candidates, the board of directors will direct the third party search firm to identify and include candidates with gender and racial/ethnic diversity as part of the retained search. The Corporate Governance Policy Statement, as well as the charters for each committee of the board, may be viewed at www.nektar.com.
The positions of Chief Executive Officer and Chairman of the board of directors are currently held by Howard W. Robin and Robert B. Chess, respectively. The board of directors believes at this time having a separate chairman provides a more effective channel for the board of directors to express its views on management, by enhancing the board of director’s oversight of, and independence from, management, and allows the Chief Executive Officer to focus more on the strategy and operations of the Company.
Lead Independent Director
Roy A. Whitfield serves as our Lead Independent Director. The board of directors believes that a robust Lead Independent Director role facilitates independent board oversight of management. In accordance with our Corporate Governance Policy Statement, the Lead Independent Director shall, among other things, (i) have authority to call meetings of the independent directors; (ii) chair meetings of the independent directors in the event the Chairman of the board of directors is not independent; (iii) serve as a liaison between the Chairman of the board and the independent directors; (iv) approve information sent to the board; (v) approve meeting agendas for the board; (vi) approve meeting schedules for the board to assure that there is sufficient time for discussion of all agenda items; and (vii) have such other duties and responsibilities as may be assigned by the board from time to time.
24
The board of directors monitors and assesses key business risks directly through deliberations of the board of directors and also by way of delegation of certain risk oversight functions to be performed by committees of the board of directors. The board of directors responsibilities include, among other matters:
Review and approval of the Company’s annual operating and capital spending plan and review of management’s updates as to the progress against the plan and any related risks and uncertainties.
Periodic consideration of the balance of risk and opportunities presented by the Company’s medium to long-term strategic plan and the potential implications of success and failure in one or more of the Company’s key drug development programs.
Regular consideration of the risks and uncertainties presented by alternative clinical development strategies.
Periodic review and oversight of information technology (e.g., cybersecurity) risks and opportunities.
Regular review of the progress and results of the Company’s clinical development programs and early research efforts including but not limited to the strengths, weaknesses, opportunities and threats for these programs.
Periodic review and oversight of material outstanding litigation or threatened litigation.
Review and approval of material collaboration partnerships for the further development and commercial exploitation of the Company’s proprietary drug development programs and technologies.
Regular review and approval of the annual corporate goals and an assessment of the Company’s level of achievement against these established goals.
Regular review of the Company’s financial position relative to the risk and opportunities for the Company’s business.
Periodic review of the Company’s intellectual property estate.
Periodic review and assessment of CEO succession planning.
Periodic review of the Company’s compensation programs.
Periodic review and assessment of the Company’s environment, social and governance-related policies.
The discussion above of risk oversight matters reviewed by the board of directors is intended to be illustrative only and not a complete list of all important matters reviewed and considered by the board of directors in providing oversight and direction for the Company’s senior management and business.
The risk oversight function of the board of directors is also administered through various board committees. The Audit Committee oversees the management of financial, accounting, internal controls, disclosure controls and the engagement arrangement and regular oversight of the independent auditors. The Audit Committee also periodically reviews the Company’s investment policy for its cash reserves, corporate insurance policies, information technology infrastructure and general fraud monitoring practices and procedures, including the maintenance and monitoring of a whistleblower hotline and the segregation of duties and access controls across various functions. To assist the Audit Committee in its risk management oversight function, the internal auditor has a direct reporting relationship to the Audit Committee. The Company’s internal audit function is focused on internal control monitoring and activities in support of the Audit Committee’s risk oversight function.
25
The Organization and Compensation Committee is responsible for the design and oversight of the Company’s compensation programs as well as succession planning for the chief executive officer position and other key executive positions. The Organization and Compensation Committee regularly considers whether the Company’s compensation policies and practices create risks that could have a material adverse impact on the Company and has concluded that they do not based on several design features of our compensation program that we believe reduces the likelihood of excessive risk-taking, including the following:
the compensation plan design provides a mix of base salary, short-term incentive compensation opportunity and equity compensation earned over multiple-year periods;
the determination of the corporate performance rating under the annual bonus plan is based on our achievement of a diversified mix of development, research, organizational and financial objectives. Thus, the achievement of any single corporate objective does not have a disproportionate impact on the aggregate annual bonus awarded;
each employee’s annual cash bonus is determined by a combination of the corporate performance rating and a subjective determination of individual performance;
the maximum payout levels for annual incentive bonuses are capped at 200% of each employee’s annual target bonus;
a substantial portion of each executive’s compensation opportunity is in the form of long-term equity incentives, which help to further align the long-term interests of our executives with those of our stockholders;
all employees are subject to our security trading policy which prohibits trading in derivative securities (i.e., puts or calls), short selling, and any trading in the Company’s securities on margin; and
each executive officer is subject to our claw-back policy which provides that any compensation received by an executive officer based upon the achievement of financial results that are subsequently revised is subject to cancellation or a reimbursement obligation.
The Nominating and Corporate Governance Committee periodically reviews the Company’s corporate governance practices, including certain risks that those practices are intended to address. The committee periodically reviews the composition of the board of directors to help ensure that a diversity of skills and experiences is represented by the members of the board of directors taking into account the stage of growth of the Company and its strategic direction.
In carrying out their risk oversight functions, the board of directors and its committees routinely request, and review management updates, reports from the independent auditors and legal and regulatory advice from outside experts, as appropriate, to assist in discerning and managing important risks that may be faced by the Company. The board of directors is committed to continuing to ensure and evolve its risk oversight practices as appropriate given the stage of the Company’s evolution as a research-based development stage biopharmaceutical company and the fast-paced changes in the biopharmaceutical industry. In that regard, in 20222023 the Company maintained a risk management committee composed of senior managers in charge of important functional areas that regularly reported to the board of directors or one of its designated committees.
As required under the NASDAQ Global SelectNasdaq Capital Market listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the board of
directors. Our board consults with counsel to ensure that its determinations are consistent with all relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent NASDAQNasdaq listing standards, as in effect from time to time.
Consistent with these standards, after review of all relevant transactions (if any) or relationships between each director, or any of his or her family members, and us, our senior management and our independent registered public accounting firm, the board has affirmatively determined that all of our directors are independent directors within the meaning of the applicable NASDAQNasdaq listing standards, except for Mr. Robin, our President and Chief Executive Officer.
26
As required under applicable NASDAQNasdaq listing standards, in the 20222023 fiscal year, our independent directors met sevenfour times in regularly scheduled executive sessions. The Lead Director presided over such sessions at which only independent directors were present.
The board of directors has three regularly constituted committees: an Audit Committee, an Organization and Compensation Committee, and a Nominating and Corporate Governance Committee. The following table provides membership and meeting information as of December 31, 2022,2023, for each of the board committees:
Name | | | Audit | | | Organization and Compensation | | | Nominating and Corporate Governance |
Jeff Ajer | | | X | | | | | X | |
Diana M. Brainard, M.D. | | | | | | | |||
Robert B. Chess | | | | | | | |||
Myriam J. Curet, M.D. | | | | | X | | | ||
Karin Eastham | | | X(1) | | | X | | | |
R. Scott Greer | | | X | | | X(1) | | | X |
Howard W. Robin | | | | | | | |||
Roy A. Whitfield | | | X | | | | | X(1) | |
Total meetings in the 2022 fiscal year | | | 5 | | | 5 | | | 3 |
| Name | | | Audit | | | Organization and Compensation | | | Nominating and Corporate Governance |
| Jeff Ajer | | | X | | | | | X | |
| Diana M. Brainard, M.D. | | | | | X | | | ||
| Robert B. Chess | | | | | | | |||
| Myriam J. Curet, M.D. | | | | | X | | | ||
| R. Scott Greer | | | X* | | | X*(1) | | | X |
| Howard W. Robin | | | | | | | |||
| Roy A. Whitfield | | | X | | | | | X* | |
| Total meetings in the 2023 fiscal year | | | 5 | | | 5 | | | 3 |
| Committee |
| (1) | Mr. Greer served as Chairperson of the Organization and Compensation until December 31, 2023. Effective January 1, 2024, Dr. Brainard serves as the Chairperson of the Organization and Compensation Committee. |
Below is a description of each standing committee of the board of directors. The board of directors has determined that each member of each committee meets the applicable rules and regulations regarding “independence” and that each member is free of any relationship that would interfere with his or her individual exercise of independent judgment with regard to us.
AUDIT COMMITTEE
The Audit Committee of the board of directors oversees our corporate accounting and financial reporting process. For this purpose, the Audit Committee performs several functions. The Audit Committee:
evaluates the performance of and assesses the qualifications of our independent registered public accounting firm;
determines whether to retain or terminate our independent registered public accounting firm or to appoint and engage a new independent registered public accounting firm;
reviews and determines the engagement of the independent auditors, including the overall scope and plans for their respective audits, the adequacy of staffing and compensation, and negotiates and executes, on behalf of the Company, engagement letters with the independent auditors;
establishes guidelines and procedures with respect to the rotation of the lead or coordinating audit partners having primary responsibility for the audit and the audit partner responsible for reviewing the audit;
reviews and approves the retention of the independent registered public accounting firm for any permissible non-audit services and, at least annually, discusses with our independent registered public accounting firm, and reviews, that firm’s independence;
obtains and reviews, at least annually, a formal written statement prepared by the independent registered public accounting firm delineating all relationships between the independent registered public accounting firm and the Company and discusses with the independent registered public accounting firm, and reviews, its independence from management and the Company;
27
reviews with the independent registered public accounting firm any management or internal control letter issued or, to the extent practicable, proposed to be issued by the independent registered public accounting firm and management’s response;
reviews with management and the independent registered public accounting firm the scope, adequacy and effectiveness of our financial reporting controls;
reviews and discusses with management, the Company’s risk management committee, the internal auditor and the independent registered public accounting firm, as appropriate, the Company’s major financial risks, the Company’s policies for assessment and management of such risks, and the steps to be taken to control such risks;
reviews and evaluates the Company’s information technology (e.g., cybersecurity) processes and risks;
establishes and maintains procedures for the receipt, retention and treatment of complaints regarding accounting, internal accounting controls or auditing matters, including procedures for the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters;
investigates and resolves any disagreements between our management and the independent registered public accounting firm regarding our financial reporting, accounting practices or accounting policies and reviews with the independent registered public accounting firm any other problems or difficulties it may have encountered during the course of the audit work;
meets with senior management and the independent registered public accounting firm in separate executive sessions;
reviews the consolidated financial statements to be included in our quarterly reports on Form 10-Q and our annual reports on Form 10-K;
discusses with management and the independent registered public accounting firm the results of the independent registered public accounting firm’s review of our quarterly consolidated financial statements and the results of our annual audit and the disclosures contained under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our periodic reports and reviews the Company’s environmental, social and governance (“ESG”) disclosures in our periodic reports;
reviews and discusses with management and the independent registered public accounting firm any material financial arrangements of the Company which do not appear on the financial statements of the Company and any significant transactions or courses of dealing with parties related to the Company;
reviews with management and the independent registered public accounting firm significant issues that arise regarding accounting principles and financial statement presentation;
oversees the Company’s internal audit function;
discusses with management and the independent registered public accounting firm any correspondence from or with regulators or governmental agencies, any employee complaints or any published reports that raise material issues regarding the Company’s consolidated financial statements, financial reporting process or accounting policies;
oversees the preparation of the Audit Committee report to be included in the Company’s annual report or proxy statement; and
reviews the Company’s investment policy for its cash reserves, corporate insurance policies, information technology infrastructure and general fraud monitoring practices and procedures, including the maintenance and monitoring of a whistleblower hotline and the segregation of duties and access controls across various functions.
The Audit Committee has the authority to retain special legal, accounting or other professional advisors to advise the committee as it deems necessary, at our expense, to carry out its duties and to determine the compensation of any such advisors.
28
Mr. Geer currently serves as the Chairperson of the Audit Committee. The board of directors annually reviews the NASDAQNasdaq listing standards definition of independence for Audit Committee members and has determined that all members of our Audit Committee are independent.
During the 20222023 fiscal year, the board of directors determined that Ms. EasthamMr. Greer also qualifies as an “Audit Committee financial expert” as defined in applicable SEC rules. The board of directors made a qualitative assessment of Ms. Eastham’s level of knowledge and experience based on a number of factors, including her formal education and experience as a Chief Financial Officer of a public reporting company. In addition to serving as the chairperson of our Audit Committee, Ms. Eastham also serves on the Audit Committees of Geron Corporation (NASDAQ: GERN), Veracyte, Inc. (NASDAQ: VCYT) and Personalis, Inc. (NASDAQ: PSNL). The board of directors does not believe that such simultaneous service impairs Ms. Eastham’s ability to effectively serve on our Audit Committee. The board of directors has also determined that Mr. Greer qualified as an “Audit Committee financial expert” as defined in applicable SEC rules. The board of directors made a qualitative assessment of Mr. Greer’s level of knowledge and experience based on a number of factors, including his formal education and prior experience as a Chief Executive Officer at a public reporting company, aand Chief Financial Officer at public reporting companies and as the chairman of public company Audit Committees. The Audit Committee has adopted a written Audit Committee charter that is available on our corporate website at www.nektar.com.
ORGANIZATION AND COMPENSATION COMMITTEE
The Organization and Compensation Committee of the board of directors administers the variable compensation programs and reviews management’s recommendations for organization structure and development of the Company. Additionally, the Organization and Compensation Committee reviews and in some cases approves the type and level of cash and equity-based compensation for officers, employees and consultants of the Company, and recommends certain compensation actions to the board of directors for review and approval. The Organization and Compensation Committee:
reviews and approves the structure and guidelines for various incentive compensation and benefit plans;
may grant equity awards under the various equity incentive compensation and benefit plans or any inducement plans established under NASDAQNasdaq Listing Rule 5635(c)(4) and IM-5635-1;
approves the compensation for the executive officers of the Company, including the President and Chief Executive Officer, and those vice-president level employees that report directly to the President and Chief Executive Officer, including, but not limited to, annual salary, bonus, equity compensation and other benefits;
recommends the compensation levels for the members of the board of directors who are not employed by us or our subsidiaries (“non-employee directors”) for approval by the independent members of the board of directors;
reviews the operation of the Company’s executive compensation programs to determine whether they remain supportive of the Company’s business objectives and are competitive relative to comparable companies and establishes and periodically reviews policies for the administration of executive compensation programs;
reviews the Company’s executive compensation arrangements to evaluate whether incentive and other forms of compensation do not encourage inappropriate or excessive risk taking and reviews and discusses, at least annually, the relationship between risk management policies and practices, corporate strategy and the Company’s executive compensation arrangements;
reviews and discusses with management and the Company’s risk management committee, as appropriate, the Company’s major risks relating to the purview of the Organization and Compensation Committee, the Company’s policies for assessment and management of such risks, and the steps to be taken to control such risks;
oversees the preparation of the Organization and Compensation Committee report to be included in the Company’s annual proxy statement and Annual Report on Form 10-K;
reviews and reassess the adequacy of the Organization and Compensation Committee charter on at least an annual basis;
reviews management recommendations on organization structure and development, including succession planning; and
reviews performance of the executive officers and vice-president level employees that report directly to the Chief Executive Officer; and
periodically reviews and discusses with management the Company’s diversity, talent, and culture strategy, which may include human capital programs and policies regarding management development, talent planning, diversity and inclusion initiatives, and employee engagement.
29
The Organization and Compensation Committee takes into account our President and Chief Executive Officer’s recommendations regarding the compensatory arrangements for our executive officers, although our President and Chief Executive Officer does not participate in the deliberations or determinations of his own compensation. In particular, the Organization and Compensation Committee considered our President and Chief Executive Officer’s recommendations for 20222023 regarding determination of annual base compensation, award of annual performance-based bonus compensation and the equity granted to our executive officers excluding himself. While the Organization and Compensation Committee considers and appreciates the input and expertise of management in making its decisions, it does ensure that an executive session where no management is present is included in the agenda for every committee meeting. The Organization and Compensation Committee’s charter gives the committee the sole authority to retain independent counsel, compensation and benefits consultants or other outside experts or advisors that it believes to be necessary or appropriate. During 2022,2023, the Organization and Compensation Committee retained Aon plc (“Aon”), a nationally recognized executive compensation consulting firm that performs compensation benchmarking, analysis and design services. RadfordAon was engaged in 20222023 to provide regulatory, legislative updates and market trend analysis, to provide analysis on our compensation programs, to provide recommendations and advice on the structure, elements and amounts of compensation provided to our non-employee directors, to provide recommendations and advice on Nektarour peer companies, to review the Compensation Discussion and Analysis, to provide pay-versus performance analysis, and to provide executive compensation analysis as needed. The Company subscribes to Aon’s general compensation survey services for ongoing trends information in addition to the executive and director compensation services it performs at the request of the Organization and Compensation Committee. In 2022,2023, we paid Aon $22,150$25,013 for provision of section specific survey services to understand the market conditions at all levels in the Company. After consideration of such services and other factors prescribed by the SEC for purposes of assessing the independence of compensation consultants, we have determined that no conflicts of interest exist between the Company and Aon (or any individuals providing such services to the committee on Aon’s behalf).
The Organization and Compensation Committee may delegate to its subcommittees such authority as it deems appropriate, except for the authority the committee is required to exercise by applicable law or regulation. The Organization and Compensation Committee has delegated certain limited authority to grant equity awards under our stock incentive plan to a committee comprised of management representatives. This committee may not approve award grants to anyone serving as an executive officer or director of the Company. Other than the authority delegated to this committee, the Organization and Compensation Committee has no current intention to delegate any of its authority to any other committee or subcommittee.
The current members of the Organization and Compensation Committee are Mr. Greer,Dr. Brainard, who chairs the committee, and Ms. EasthamMr. Greer and Dr. Curet. The board of directors annually reviews the NASDAQNasdaq listing standards definition of independence for Organization and Compensation Committee members and has determined that all members of our Organization and Compensation Committee are independent. The Organization and Compensation Committee charter can be found on our corporate website at www.nektar.com.
NOMINATING AND CORPORATE GOVERNANCE COMMITTEE
The Nominating and Corporate Governance Committee:
establishes criteria for board membership, including standards for independence, and considers and assesses the independence of the directors;
evaluates board composition and performance;
identifies, reviews and recommends the board of directors’ selected candidates to serve as directors;
considers stockholder recommendations for director nominations and other proposals submitted by stockholders;
reviews the adequacy of, and compliance with, our Code of Business Conduct and Ethics;
administers and oversees all aspects of our corporate governance functions on behalf of the board of directors;
monitors regulatory and legislative developments in corporate governance, as well as trends in corporate governance practices, and makes recommendations to the board of directors regarding the same;
30
reviews and discusses with management and the Company’s risk management committee, as appropriate, the Company’s major risks relating to the purview of the nominating and corporate government committee, the Company’s policies for assessment and management of such risks, and the steps to be taken to control such risks;
establishes and oversees procedures for the receipt, retention and treatment of complaints received by the Company with respect to legal and regulatory compliance (except for compliance relating to accounting, internal accounting controls, auditing matters and financial disclosure and reporting);
provides recommendations to the board of directors to establish such special committees as may be desirable or necessary from time to time in order to address ethical, legal, business or other matters that may arise; and
assist in the development and recommend to the board of directors, as appropriate, policies and programs with respect to ESG matters relevant to the company’s business.
The Nominating and Corporate Governance Committee believes that candidates for director should possess the highest personal and professional ethics, integrity and values, be committed to represent our long-term interests and those of our stockholders, possess diverse experience at policy-making levels in business, science and technology, possess key personal characteristics such as strategic thinking, objectivity, independent judgment, intellect and the courage to speak out and actively participate in meetings, as well as have sufficient time to carry out the duties and responsibilities of a board member effectively.
Candidates for director nominees are reviewed in the context of the current composition of the board, our operating requirements and the long-term interests of stockholders. In conducting this assessment, the committee considers diversity, age, skills and such other factors as it deems appropriate given our current needs and those of our board to maintain a balance of knowledge, experience and capability. The Nominating and Corporate Governance Committee also periodically reviews the overall effectiveness of the board, including board attendance, level of participation, quality of performance, self-assessment reviews and any relationships or transactions that might impair director independence. In the case of new director candidates, the Nominating and Corporate Governance Committee also determines whether the nominee must be independent for NASDAQNasdaq purposes, which determination is based upon applicable NASDAQNasdaq listing standards, applicable SEC rules and regulations and the advice of counsel, if necessary. The committee then uses its network of contacts to compile a list of potential candidates, but may also engage, if it deems appropriate, a professional search firm. The committee conducts any appropriate and necessary inquiries into the backgrounds and qualifications of possible candidates after considering the function and needs of the board. The Nominating and Corporate Governance Committee meets to discuss and consider such candidates’ qualifications and then selects a nominee for recommendation to the board by majority vote. We have paid fees to third party search firms in the past to assist in our process of identifying or evaluating director candidates.
The Nominating and Corporate Governance Committee of our board of directors will consider for nomination any qualified director candidates recommended by our stockholders. Any stockholder who wishes to recommend a director candidate is directed to submit in writing the candidate’s name, biographical information, relevant qualifications and other information required by our bylaws to our Secretary at our principal executive offices before the deadline set forth in our bylaws. All written submissions received from our stockholders will be reviewed by the Nominating and Corporate Governance Committee at the next appropriate meeting. The Nominating and Corporate Governance Committee will evaluate any suggested director candidates received from our stockholders in the same manner as recommendations received from management, committee members or members of our board.
The current members of the Nominating and Corporate Governance Committee comprise Mr. Whitfield, who chairs the committee, Mr. Ajer and Mr. Greer. The board of directors annually reviews the NASDAQNasdaq listing standards definition of independence for the Nominating and Corporate Governance Committee and has determined that all members of our Nominating and Corporate Governance Committee are independent. The Nominating and Corporate Governance Committee charter can be found on our corporate website at www.nektar.comwww.nektar.com..
31
BOARD COMPOSITION
The current members of our board of directors represent a desirable mix of backgrounds, skills and experiences, and are all believed to share the key personal characteristics described above. Below are some of the specific experiences and skills of our directors.
Jeff Ajer
Mr. Ajer, has more than 25 years of biotechnology industry experience within rare disease and specialty medicine. Mr. Ajer currently serves as Executive Vice President and Chief Commercial Officer at BioMarin Pharmaceutical Inc. Mr. Ajer recently served on the board of directors of True North Therapeutics. Mr. Ajer has extensive knowledge and expertise of the biotechnology industry.
Diana M. Brainard, M.D.
Dr. Brainard has more than 20 years of experience in the biopharmaceutical industry and academic medicine and has authored more than 200 publications. She currently serves as the Chief Executive Officer and a member of the board of directors of AlloVir, Inc., a public reporting company.
Robert B. Chess
Mr. Chess is our Chairman and former President and Chief Executive Officer and has a deep understanding of our business. Having founded and led private and public companies, Mr. Chess has strong experience leading growing companies in our industry. Due to his long association with the Company as a director and senior executive leader at various times, he possesses significant knowledge and perspective on the history and development of the Company. Mr. Chess is a prominent participant in our industry, was a long-time member of the board of our industry association, and is on the board of trustees and faculty of leading academic institutions.
Myriam J. Curet, M.D.
Dr. Curet has over 20 years of experience in the biopharmaceutical industry and faculty positions, and currently serves as the Executive Vice President and Chief Medical Officer of Intuitive Surgical. Dr. Curet has held senior executive positions and has served as Vice President and Chief Medical Officer in November 2017, as the Chief Medical Advisor for Intuitive Surgical from December 2005 to February 2014 and as Intuitive Surgical’s Senior Vice President and Chief Medical Officer from February 2014 to November 2017. Dr. Curet also has a faculty position as Professor of Surgery at Stanford University School of Medicine. Since October 2010, she has served as a Consulting Professor of Surgery at Stanford University with a part time clinical appointment at the Palo Alto Veteran’s Administration Medical Center. She was also on the faculty at the University of New Mexico for six years prior to joining the Stanford University in 2000.
R. Scott Greer
Mr. Greer has a proven track record as an entrepreneur and senior executive with extensive experience in the biotechnology industry, most recently with Abgenix, Inc., until its acquisition by Amgen, Inc. in 2006. Mr. Greer has held senior executive and finance positions at other companies in our industry and currently servespreviously served as a director of several other companies in the biopharmaceutical and medical device industries, and has servedincluding as the Chairman of the Board of several companies. He possesses strong expertise in biotech industry strategy, business models, and finance and has served on compensation, governance and audit committees.
Howard W. Robin
Mr. Robin is our President and Chief Executive Officer. Mr. Robin has over 25 years of experience in the pharmaceutical and biotechnology industries in a variety of roles of increasing responsibility and, prior to becoming our chief executive officer, was the chief executive officer and president and a director of Sirna Therapeutics, a development stage biotechnology company. The board of directors has determined that Mr. Robin’s position as president and chief executive officer provides him with important insight into the Company’s opportunities, risks, strengths and weaknesses, as well as its organizational and operational capabilities, which is valuable to the board of directors in making strategic decisions and performing its oversight responsibilities.
32
Roy A. Whitfield
Mr. Whitfield has a strong strategy development and leadership background in the biotechnology and medical industries. He is a former strategy consultant from a major consulting firm, was the founder and chief executive officer of a public biotechnology company and has held executive positions in various segments of the health care industry. He has extensive corporate governance experience through his service on other public company boards in the pharmaceutical and life sciences industries.
The following table sets forth certain diversity statistics as self-reported by the current members of our Board of Directors as of April 10, 2022.8, 2024.
Board Diversity Matrix
| | Board Size: | | ||||||||||||
| | Total Number of Directors | | | 8 | | |||||||||
| | Gender: | | | Female | | | Male | | | Non-Binary | | | Did not disclose Gender | |
| | Number of directors based on gender identity | | | 3 | | | 5 | | | — | | | — | |
| | Number of directors who identify in any of the categories below: | | ||||||||||||
| | African American or Black | | | — | | | — | | | — | | | — | |
| | Alaskan Native or American Indian | | | — | | | — | | | — | | | — | |
| | Asian | | | — | | | — | | | — | | | — | |
| | Hispanic or Latinx | | | 1 | | | — | | | — | | | — | |
| | Native Hawaiian or Pacific Islander | | | — | | | — | | | — | | | — | |
| | White | | | 3 | | | 5 | | | — | | | — | |
| | Two or More Races or Ethnicities | | | 1 | | | — | | | — | | | — | |
| | LGBTQ+ | | | — | | |||||||||
| | Board Size: | | ||||||||||||
| | Total Number of Directors | | | 7 | | |||||||||
| | Gender: | | | Female | | | Male | | | Non-Binary | | | Did not disclose Gender | |
| | Number of directors based on gender identity | | | 2 | | | 5 | | | — | | | — | |
| | Number of directors who identify in any of the categories below: | | ||||||||||||
| | African American or Black | | | — | | | — | | | — | | | — | |
| | Alaskan Native or American Indian | | | — | | | — | | | — | | | — | |
| | Asian | | | — | | | — | | | — | | | — | |
| | Hispanic or Latinx | | | 1 | | | — | | | — | | | — | |
| | Native Hawaiian or Pacific Islander | | | — | | | — | | | — | | | — | |
| | White | | | 1 | | | 5 | | | — | | | — | |
| | Two or More Races or Ethnicities | | | — | | | — | | | — | | | — | |
| | LGBTQ+ | | | — | | |||||||||
The board of directors will consider any written or electronic communication from our stockholders to the board, a committee of the board or any individual director. Any stockholder who wishes to communicate to the board of directors, a committee of the board or any individual director should submit written or electronic communications to our Secretary at our principal executive offices, which shall include contact information for such stockholder. All communications from stockholders received will be forwarded by our Secretary to the board of directors, a committee of the board or an individual director, as appropriate, on a periodic basis, but in any event no later than the board of directors’ next scheduled meeting. The board of directors, a committee of the board, or individual directors, as appropriate, will consider and review carefully any communications from stockholders forwarded by our Secretary.
We have adopted a code of business conduct and ethics that applies to all employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. The code of business conduct and ethics is available on our website at http://ir.nektar.com/governance. Amendments to, and waivers from, the code of business conduct and ethics that apply to any director, executive officer or persons performing similar functions will be disclosed at the website address provided above and, to the extent required by applicable regulations, on a Current Report on Form 8-K filed with the SEC.
33
As of December 2022,2023, the Organization and Compensation Committee consisted of three independent directors: Dr. Brainard Mr. Greer, and Dr. Curet, and Ms. Eastham.Curet. No director who served on the Organization and Compensation Committee in 20222023 was, or has been, an officer or employee of us, nor has any director had any relationships requiring disclosure under the SEC rules regarding certain relationships and related-party transactions. None of our executive officers served on the board of directors or the Organization and Compensation Committee (or other board committee performing equivalent functions) of another entity, one of whose executive officers served on our board of directors or Organization and Compensation Committee.
Each of our non-employee directors participates in our Amended and Restated Compensation Plan for Non-Employee Directors (the “Director Plan”). Only our non-employee directors are eligible to participate in the Director Plan. The following table shows compensation awardedthe fees earned or paid to our non-employee directors for the fiscal year ended December 31, 2022.2023.
Name(1) (a) | | | Fees Earned or Paid in Cash ($) (b) | | | Stock Awards ($)(2) (c) | | | Option Awards ($)(3) (d) | | | All Other Compensation (e) | | | Total ($) (f) |
Jeff Ajer | | | 93,250 | | | 35,394 | | | 46,849 | | | 0 | | | 175,493 |
Diana Brainard, M.D. | | | 65,000 | | | 35,394 | | | 46,849 | | | 0 | | | 147,243 |
Robert Chess | | | 131,500 | | | 35,394 | | | 46,849 | | | 0 | | | 213,743 |
Myriam Curet, M.D. | | | 76,000 | | | 35,394 | | | 46,849 | | | 0 | | | 158,243 |
Karin Eastham | | | 122,750 | | | 35,394 | | | 46,849 | | | 0 | | | 204,993 |
R. Scott Greer | | | 115,500 | | | 35,394 | | | 46,849 | | | 0 | | | 197,743 |
Roy A. Whitfield | | | 126,750 | | | 35,394 | | | 46,849 | | | 0 | | | 208,993 |
Name(1) (a) | | | Fees Earned or Paid in Cash ($) (b) | | | Stock Awards ($) (c) | | | Option Awards ($)(2) (d) | | | All Other Compensation (e) | | | Total ($) (f) |
| Jeff Ajer | | | 92,000 | | | — | | | 42,220 | | | — | | | 134,220 |
| Diana Brainard, M.D. | | | 70,500 | | | — | | | 42,220 | | | — | | | 112,720 |
| Robert Chess | | | 134,000 | | | — | | | 42,220 | | | — | | | 176,220 |
| Myriam Curet, M.D. | | | 76,000 | | | — | | | 42,220 | | | — | | | 118,220 |
Karin Eastham(3) | | | 62,000 | | | — | | | — | | | — | | | 62,000 |
| R. Scott Greer | | | 128,000 | | | — | | | 42,220 | | | — | | | 170,220 |
| Roy A. Whitfield | | | 128,000 | | | — | | | 42,220 | | | — | | | 170,220 |
| (1) | Mr. Robin, our President and Chief Executive Officer, is not included in this table as he was an employee of the Company in |
| (2) |
| Amounts reported represent the aggregate grant date fair value of option awards computed in accordance with FASB ASC Topic 718, which excludes the effects of estimated forfeitures. For a complete description of the assumptions made in determining the valuation, please refer to Note |
| (3) | Ms. Eastham’s term as a director expired on June 8, 2023. As of December 31, 2023, Ms. Eastham has 82,200 stock options outstanding. |
The 20222023 compensation for the Company’s non-employee directors was recommended by the Organization and Compensation Committee to the Board following the receipt of a report from our independent compensation consultant, which in 20222023 was Aon, plc, which contained an analysis of prevailing market practices regarding levels and types of non-employee director compensation, including the non-employee director compensation practices of our 20222023 peer group set forth below in the section entitled “Compensation Discussion and Analysis”, and a comparative assessment of our non-employee director compensation to such peers and market practices. On at least an annual basis, qualified experts deliver a presentation to the Organization and Compensation Committee about recent developments and best practices related to non-employee director compensation.
Effective January 1, 20222023 the annual retainer for non-employee directors was $65,000 (“Annual Retainer”) for up to 13 board meetings in each calendar year, after which each member received compensation in the amount of $2,000 for attendance at each subsequent Board Meeting (whether in-person or remotely). In addition to the Annual Retainer, the Chairperson of the Board of Directors received an additional annual retainer of $50,000 for a total of $115,000, and the Independent Lead Director of the Board of Directors received andan additional annual retainer of $25,000 for a total of $90,000. The annual retainer amount was $33,000 for the Chair of the Audit Committee and $13,000 for members other than the Chair for up to 9 Audit Committee meetings in each calendar year, after which each member received compensation in the amount of $1,750 for attendance at each subsequent Audit Committee meeting (whether in-person or remotely) in the calendar year, $26,000 for the Chair of the Compensation Committee and $11,000 for members other than the Chair of the Compensation Committee for up to 8 Compensation Committee meetings in each calendar year, after which each member received compensation in the amount of $1,750 for attendance at each subsequent Compensation Committee meeting (whether in-person or remotely) in the calendar year, and $20,000 for the Chair of the
Governance Committee and $9,000 for members other than the Chair of the Governance Committee for up to 6 Governance Committee meetings in each calendar year, after which each member received compensation in the amount of $1,750 for attendance at each subsequent Governance Committee meeting (whether in-person or remotely) in the calendar year. The Chair and members of any new committees would receive an additional annual retainer of $5,000, unless otherwise specified in the resolutions duly forming such new committee.
35
In September of each year, each non-employee director is eligible to receive an equity award consisting of either all stock options or a combination of stock options and RSUs, as determined by the board of directors. These equity awards vest over a period of one year (monthly for stock options and upon the anniversary date for RSUs) and include a number of shares as determined annually by the board of directors. In September 20222023 our then-serving non-employee directors received 20,40085,000 stock options and 10,200 RSUs.options. No RSUs were granted. Upon initial appointment to the board of directors, each non-employee director is eligible to receive an equity award consisting of either all stock options or a combination of stock options and RSUs. These initial equity awards vest over a period of three years from the date of appointment and will be at a level based on 180% of the most recent annual equity compensation grant to non-employee directors, as determined annually by the board of directors. The exercise price of stock options granted is equal to the closing price of the Company’s common stock on the grant date. Following completion of a non-employee director’s service on the board of directors, his or her stock options will remain exercisable for a period of eighteen months (or, if earlier, the end of the maximum term of the option). The term of stock options granted to non-employee directors is eight years. In the event of a change of control, the vesting of each option or RSU award held by each non-employee director will accelerate in full as of the closing of such transaction. In the event of death or disability, each RSU of the non-employee director will vest immediately. In the event a non-employee director retires from the board of directors at the next annual stockholder’s meeting, his or her RSU awards vest on a pro-rata basis.
The Director Plan includes non-binding ownership guidelines for non-employee directors stating that each non-employee director should own shares of our common stock equal to at least three times the value of the annual board cash retainer. The minimum stock ownership level was to be achieved by each non-employee director within five years of the date of his or her first appointment to the board of directors. AsEach of December 31, 2022, eachour non-executive directors with over five years of service on the board of directors has met the ownership guidelines previously, and all but one non-employee director metcurrently meets the minimum stock ownership guidelines or wasis within the five-year grace period provided by the plan. The board of directors understands that the recent unexpected drop in the share price of our common stock has caused a temporal diminution in the value of the common stock owned by one non-executive director so as to currently equal less than three times the value of the annual board cash retainer. As allowed by our ownership guidelines, the board of directors will continue to monitor the situation and, if necessary, develop an alternative stock ownership guideline that reflects the intention of these guidelines. Our 2017 Plan also limits the aggregate value of cash compensation and the grant date fair value (computed in accordance with generally accepted accounting principles) of shares of Common Stock that may be paid or granted during any calendar year to any non-employee director with a maximum of $1,200,000 for existing non-employee directors and $2,200,000 for new non-employee directors.
COMPENSATION DISCUSSION AND ANALYSIS
The following Compensation Discussion and Analysis is designed to provide our stockholders with an understanding of our executive compensation philosophy and decision-making process. Pursuant to the rules promulgated by the SEC, it discusses the principles underlying the structure of the compensation arrangements of our named executive officers (collectively, “NEOs”) identified in the table below. Unless noted otherwise, any reference within the Compensation Discussion and Analysis to decisions made by the board of directors refers to the decisions made by the independent members of the board of directors only.
Name | | | Title |
Howard W. Robin | | | President and Chief Executive Officer |
| | | Interim Chief Financial Officer | |
| Mark A. Wilson | | | Chief Legal Officer |
| Jonathan Zalevsky, Ph.D. | | | Chief Research and Development Officer |
Jillian B. Thomsen(2) | | | Former Chief Financial Officer and Chief Accounting Officer |
| (1) | Ms. |
| (2) | Ms. Thomsen served as our Chief Financial Officer and Chief Accounting Officer |
Executive Summary
We are a clinical stage, research-based drug discovery biopharmaceuticalbiotechnology company focused on discovering and developing innovative medicines in the field of immunotherapy. Within this growing field, we direct our efforts toward creating new immunomodulatory agents that selectively induce, amplify, attenuate or prevent immune responses in order to achieve desired therapeutic outcomes. We apply our deep understanding of immunology and unparalleled expertise in polymer chemistry to create innovative drug candidates and use our drug development expertise to advance these molecules through preclinical and clinical development.
In autoimmune diseases, we focus on addressing imbalances in the immune system to restore the body’s self-tolerance mechanisms and to achieve immune homeostasis. Our lead investigational drug candidate rezpegaldesleukin is designed to increase the function of regulatory T cells, powerful inhibitory immune cells, in order to help rebalance the body’s immune system. Rezpegaldesleukin has advancedsystem in people with autoimmune disorders and inflammatory diseases. In late October 2023, we initiated a Phase 2b clinical study of rezpegaldesleukin in patients with moderate-to-severe atopic dermatitis, and in March 2024, we initiated a Phase 2b clinical study of rezpegaldesleukin in patients with severe to Phase 2 developmentvery severe alopecia areata. We developed rezpegaldesleukin and own full rights to this drug candidate. Although we previously entered into a license agreement with Eli Lilly and Company (“Lilly”) in various indications, including atopic dermatitis. On2017 to develop and commercialize rezpegaldesleukin, on April 27, 2023, we announced that we would be regaining the full rights to rezpegaldesleukin. In addition to rezpegaldesleukin, from Eli Lilly.we are advancing another important immunology program. We planbelieve that our preclinical tumor necrosis factor receptor type II (TNFR2) agonist asset, NKTR-0165, is a potentially unique bivalent antibody that selectively stimulates TNFR2 receptor activity, which drives immunoregulatory function and can provide a direct protective effect for tissue cells. TNFR2 is highly expressed on T regulatory cells (Tregs), neuronal cells and endothelial cells and has been shown to initiate a Phase 2b studypotentiate the suppressive effects and overall functional properties of rezpegaldesleukinTregs. Our focus is on TNFR2 antibody candidates that show selective Treg cell binding and signaling profiles that may be potentially developed for treatment of autoimmune diseases, such as ulcerative colitis, multiple sclerosis and vitiligo. We are currently conducting Investigational New Drug (IND) enabling studies with the goal of targeting an IND submission in
37
immunological memory, which may lead to sustained and durable anti-tumor immune response. Our development strategy forWe are continuing select developmental studies of NKTR-255 is focused on three therapeutic areas: to enhance response to antibody-dependent cellular cytotoxicity mediated therapies by restoring natural killer cells, to improve CAR-Tin combination with cell persistency in cellular therapies and checkpoint inhibitors while we evaluate additional strategic partnership pathways for the program.
In April of 2022 and 2023, we completed significant strategic restructuring and reorganization actions that prioritized key research and development efforts that will be most impactful to augment responsethe Company’s future. We believe these strategic changes were necessary in order to checkpoint inhibitors.build our remaining pipeline programs and to situate the Company in a position to allow for future growth and value creation to our shareholders. We refocused the company’s development pipeline on immunology and inflammation, with our primary near-term goal to advance rezpegaldesleukin to meaningful data catalysts in the first half of 2025. Our 2023 strategic reprioritization and cost restructuring plan (the “2023 Restructuring Plan”) is intended to help extend the Company’s cash runway to mid-2026.
Our dedicated employees, scientists, executive team and board of directors are committed to our mission to discover and develop novel therapies to treat autoimmune disorders and cancer and some of the significant accomplishments the Company achieved in 20222023 are highlighted below:
In late 2023, as wellwe also began designing a Phase 2b trial of rezpegaldesleukin in alopecia areata and in March 2024, we initiated a Phase 2b clinical study of rezpegaldesleukin in patients with severe to very severe alopecia areata. We continue to explore other auto-immune indications for the development plan forof rezpegaldesleukin.
We executed a new strategycontinued to pursue NKTR-255 as a potentiator in cellular therapies. We initiated two ongoing investigator sponsoredconduct developmental studies to study the pharmacodynamics and safety of NKTR-255 in combination with CAR-T cell therapy. In December 2022,therapies and checkpoint inhibitors while we initiated aevaluate additional strategic partnership pathways for the program. We completed enrollment for the Nektar-sponsored Phase 2/3 study (currently in the Phase 2 portion) to evaluate NKTR-255 following Yescarta® or Breyanzi® CD19 CAR-T cell therapy in patients with large B-cell lymphoma. We also continued our oncology clinical collaboration with Merck KGaA to evaluate the maintenance regimen of NKTR-255 in combination with avelumab, a PD-L1 inhibitor, in patients with locally advanced or metastatic urothelial carcinoma in the Phase II JAVELIN Bladder Medley study. We expect to receive topline data from the study in the second half of 2024.
In September 2023, we entered into a new clinical study collaboration with AbelZeta Pharma, Inc. (AbelZeta) (formerly known as CBMG Holdings) to study NKTR-255 in combination with its C-TIL051, a tumor-infiltrating lymphocyte (TIL) therapy, in advanced non-small cell lung cancer (NSCLC) patients that are relapsed or refractory to anti-PD-1 therapy. Under the collaboration, we will contribute NKTR-255 and AbelZeta will add NKTR-255 to its ongoing AbelZeta-sponsored Phase 1 clinical trial.
38
We further advanced our research program focused on developing a tumor necrosis factor (TNF) receptor 2 (TNFR2) agonist antibody. TNFR2 signaling drives immunoregulatory function and can provide a direct protective effect for tissue cells. Our focus is on TNFR2 antibody candidates that show selective regulatory T cell binding and signaling profiles that may be developed for treatment of autoimmune diseases. In connection with this program, we are targeting our efforts to support the filing of an Investigational New Drug (IND) for a lead TNFR2 agonist antibody, candidate.NKTR-1065. We are carrying out IND enabling studies for this program in 2024, after having exercised an option to gain an exclusive license to specified agonistic antibodies and other materials that were developed pursuant to a research collaboration and license option agreement we entered into with Biolojic Design, Ltd. in 2021.
We successfully completed allimplemented the 2023 Restructuring Plan to enable us to focus on our pipeline of immunology and inflammation programs. We undertook several cost-saving measures and reduced our commercial PEG manufacturing obligations despite the significantSan Francisco-based workforce reduction and continued global supply chain shortages.
We ended 20222023 with approximately $505.0$329.4 million in cash and investments in marketable securities and following implementation of the 2023 Restructuring Plan, we expect to extend our cash runway through the middle of 2026.
We believe our business strategy has the potential to create significant value to our stockholders if one or more of our current drug candidates demonstrates positive clinical results, receives regulatory approval in one or more major markets and achieves commercial success.
Compensation Highlights
We consider the intellectual capital of our employees to be an essential driver of our business and key to the successful execution of our strategy. Accordingly, we aim to attract and retain high-performing executives, and our executive officer compensation program is designed to reward achievement of important business and strategic goals and to pay for performance. We also recognize that the biotechnology industry is characterized by high stock price volatility, uncertainty and intense competition and that the Company’s stock price at any given point in time may not be reflective of the Company’s accomplishments and performance over a sustained period. We use a variety of performance-based compensation elements, including long-term equity awards that have both time and performance-based vesting criteria and annual cash incentives that are based on both corporate performance and an individual’s performance, in order to align our NEO’sNEOs’ interests with those of our stockholders. We believe this incentivizes our management team to invest in and to develop strategies that will create long-term growth and value for our stockholders.
In 2022,2023, our board of directors and Organization and Compensation Committee took the following key compensation actions:
| • | Base salaries – No merit adjustments were made to executive base salaries, |
| • | Alignment of CEO bonus to |
| • | Annual cash incentives for NEOs – Our NEOs, |
| • | Long-term Incentives – |
| • | Performance-Based Compensation – We believe that a significant portion of our |
| • | Peer Group Alignment – |
| • | Enhanced disclosures – In order to provide transparency for our stockholders and assist them in understanding the alignment between NEO pay and performance of corporate objectives, we have continued to provide enhanced disclosures concerning long-term incentive and short-term incentive grants, including specific targets and achievements met by the Company. |
How Our Pay Program Works
We believe that the design and structure of our pay program, and in particular our incentive plans, supports our business strategy while successfully aligning executive focus and interests with those of our stockholders. The business achievements described above would not be possible without our talented executive team. We believe that each element chosen for our executive compensation program helps us to achieve our compensation objectives. For example, we believe that performance-based, short-term cash incentive opportunities in combination with performance-based, long-term equity incentive awards is the best way to align our executives’ interests with those of our stockholders. We also believe that the long-term vesting schedules applicable to equity awards also serve as a significant retention incentive as well as a focus on building long-term stockholder value.
We use the following framework to achieve our pay program objectives:
Base Salary | | | Base salaries are set to be competitive within our industry with consideration for, among other things, an individual’s responsibilities, market data and individual contribution. In |
Annual Cash Incentives | | | Annual incentives are intended to motivate and reward executives for the achievement of important short-term goals and milestones that we believe contribute to the creation of long-term stockholder value. In |
| | | ||
Long-Term Equity Incentives | | | Our long-term incentives, which include performance-based vesting equity awards and time-based vesting equity awards, are intended to motivate executives to deliver long-term stockholder value and to retain our talented executive team. In |
Target Pay Mix
Consistent with our pay for performance philosophy, we believe that the largest componenta significant portion of overall directour executives’ compensation for our NEOs should be performance-based; therefore, in 2022 approximately 87% of total direct“at-risk” and performance-based. We emphasize performance-based compensation providedthat motivates our executives to our NEOs, on average, was tied to Companyachieve important corporate goals by providing long-term equity incentives that have performance-based criteria and individual performance objectives. We believe this is appropriate, as an executive’s ability to impact operational performance increases, so should the proportion of each individual’s at-risk compensation. Target long-term incentive compensation grows proportionately as job responsibilities increase, which encourages our NEOs to focusannual cash incentives that are awarded based on the Company’s long-term success and long-term stockholder value creation.achievement of specific annual objectives. In 2022,2023, approximately 90%59% of total target direct compensation to Mr. Robin was linked directly to the performance of the company (performance-based), and therefore not guaranteed.
40
Relationship between Company Performance and Executive Pay
The biotechnology industry is characterized by a higher risk profile and by more binary business outcomes than other, more traditional industries. This historically has led to high stock volatility for biotechnology companies. The graph below demonstrates that even with high levels of volatility in stock price, total compensation for Mr. Robin is generally aligned with our stock price performance over the past five years:
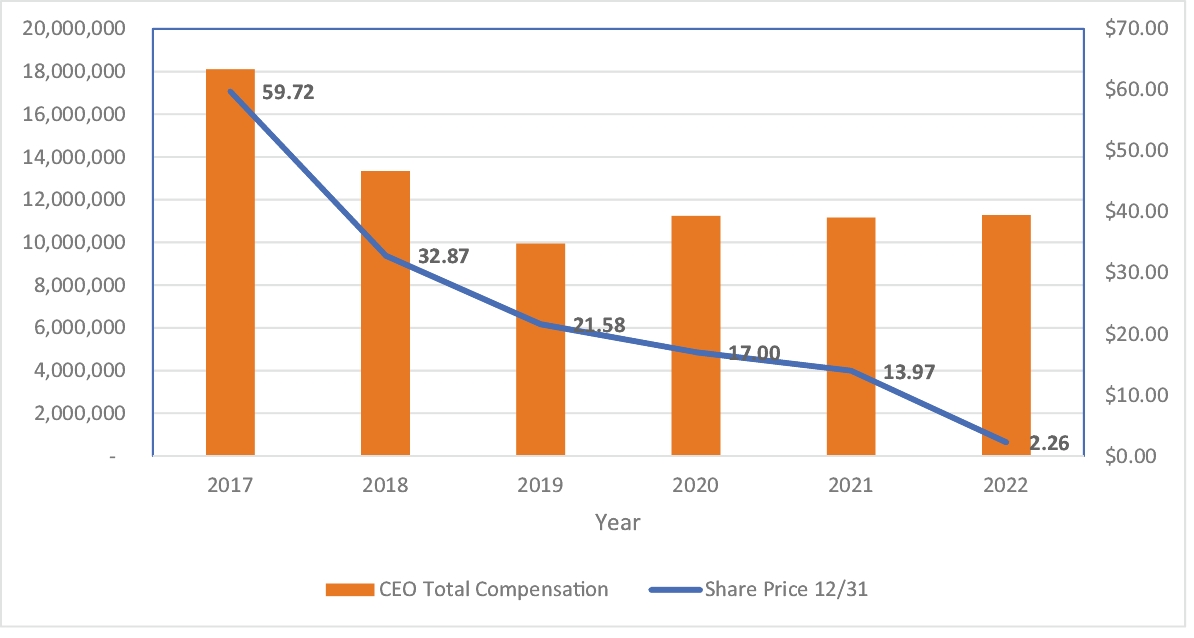

COMPENSATION GOVERNANCE PRACTICES
Our Organization and Compensation Committee is responsible for oversight of our compensation program. A significant part of this oversight is aligning management interests with our business strategy and long-term goals, as well as the interests of our shareholders, while also mitigating excessive risk-taking. We continually take steps to strengthen and improve our executive compensation policies and practices. Highlights of our current practices include:
What We Do | | | What We Don’t Do |
Deliver executive compensation primarily through performance-based pay | | | Hedging transactions, share pledging, or short sales by employees or directors |
| | | ||
| Utilize equity awards of which a majority are performance-based and designed to deliver long term stockholder value | | | Permit repricing of stock options without shareholder approval |
| | | ||
| Have a clawback policy covering cash and equity incentive compensation | | | Provide excessive perquisites |
| | | ||
| Conduct a regular peer group review | | | Provide funded pension or retirement plans (other than a matching contribution of up to $12,000 for 401(k) plan participants that we make available to all employees) |
| | |
41
| What We Do | | | What We Don’t Do |
| Use a double trigger in our Change-in-Control (CIC) severance plan | | | Provide excise tax gross-ups on CIC payments |
| | | ||
| Have stock ownership guidelines applicable to our senior executive officers | | | Accelerate vesting of equity awards on termination (unless in connection with a change of control of the Company) |
| | | ||
| Utilize independent compensation consulting firm | | | Include the value of equity awards in severance calculations |
| | | ||
| Conduct a regular review of share utilization for equity compensation | | | Enter into fixed employment terms |
| | | ||
| Design our programs to mitigate undue risk | | | |
| | | ||
| Conduct an annual say-on-pay vote and regular shareholder outreach | | |
ROLE OF STOCKHOLDER SAY-ON-PAY VOTES AND ENGAGEMENT
We provide our stockholders with the opportunity to cast an annual advisory vote to approve our executive compensation program (referred to as a “say-on-pay vote”). At our annual meeting of stockholders held in June 2022,2023, approximately 83%85% of the votes present or represented voted in favor of the proposal. After considering the 20222023 say-on-pay vote, the Organization and Compensation Committee reaffirmed the design and elements of our executive compensation program. As noted above, however, no executive received any new equity grant or merit increase to base cash compensation as part of our annual performance review process in 2022. The board of directors and the Organization and Compensation Committee will continue to consider the outcome of our say-on-pay proposals and direct stockholder feedback when making future compensation decisions for the NEOs. Currently, we hold a non-binding advisory vote on the compensation of our NEOs every year. An advisory vote on the frequency of future advisory votes on the compensation paid to our NEOs is required to be held at least once every six years. At our annual meeting of stockholders held in June 2023, approximately 98% of the votes present or represented voted in favor of having the advisory vote held every year. The Company will continue to hold a non-binding advisory vote on the compensation of our NEOs every year.
Engaging with our stockholders helps us to understand how they view us, assists in setting goals and expectations for our performance, and identifies any emerging issues that may affect our strategies, corporate governance, compensation practices or other aspects of our operations. Throughout the year, members of Investor Relations and other subject-matter experts within the Company engage with our stockholders to remain well-informed regarding their perspective on current issues, as well as to address any questions or concerns. These teams serve as liaisons between stockholders, members of senior management and the Board. Additionally, our stockholder and investor outreach includes investor road shows, analyst meetings, and investor conferences and meetings. We seek stockholder feedback on executive compensation, governance and other matters throughout the year, concentrating our efforts on our largest stockholders.
COMPENSATION PROGRAM OBJECTIVES AND PHILOSOPHY
In order to continue the execution and growth of our business as described above, we believe that it is vital that we continue to retain and attract highly experienced and skilled senior leadership by offering competitive base compensation and benefits, significant performance-based incentives, and the potential for long-term equity compensation. Our goal is to structure a meaningful portion of executive compensation such that it will only have value if the senior leadership is successful in building significant long-term value for our business and our stockholders.
Our current compensation programs for the NEOs are determined and approved by the Organization and Compensation Committee of our board of directors. The Organization and Compensation Committee takes into account Mr. Robin’s recommendations regarding the compensatory arrangements for our executive officers, although Mr. Robin does not participate in the deliberations or determinations of his own compensation. The other NEOs diddo not have any substantive role in determining or recommending the form or amount of compensation paid to any of our executive officers. Our current executive compensation programs are intended to achieve the following four fundamental goals and objectives to:
Incentivize and reward sustained long-term performance by aligning significant elements of executive compensation with long-term stockholder value creation;
Attract and retain an experienced, highly qualified and motivated executive management team to lead our business;
Provide economic rewards for achieving high levels of performance and individual contribution; and
Pay compensation that is competitive, taking into account the experience, skills and performance of the executives required to build and maintain the organization necessary to support our mission to be a leading research-based development stage biopharmaceutical company that discovers and develops innovative medicines in areasthe field of high unmet medical need.immunotherapy.
When structuring our executive compensation programs designed to achieve our goals and objectives, we are guided by the following philosophies:
| • | Alignment with Long-Term Stockholder Value Creation. Our compensation model is designed to align the economic interests of our executives with long-term stockholder value creation. Under our program, in |
| • | Pay for Performance. The objective of our executive compensation program is to deliver compensation above industry median for exceptional performance and deliver compensation below the median in performance periods where the Company does not perform well. We will also link each NEOs’ annual merit equity award to an assessment of individual performance or the achievement of what we believe to be rigorous and objective performance achievement milestones or criteria (such as filing of an IND for an investigation drug candidate). |
| • | Total Rewards Program. The total compensation program must balance pay for performance elements with selected static non-performance-based elements in order to create a total rewards program that is competitive and will help us attract and retain highly qualified and motivated executives. |
| • | Flexible Approach. The level of compensation provided to executives must take into account each executive’s role, experience, tenure, performance and expected contributions to our future success. |
| • | Focus on Achievement of Fundamental Business Goals. The compensation program should be structured so that executives are appropriately incentivized to achieve our short- and long-term goals that are viewed as fundamental to driving long-term value in our business. |
We designed our total compensation program to combine short- and long-term components, cash and equity, and fixed and contingent payments, in proportions that we believe are appropriate to achieve each of our fundamental compensation philosophies as described above. It was our intent to design the structure of the compensation program to provide appropriate incentives to reward executives for achieving our long-term goals and objectives, some of the most important of which are building and advancing a robust drug candidate
pipeline, including conducting clinical trials, entering into new collaboration partnerships and executing on our
43
current collaborations, increasing the skill level and efficiency of our organization and improving our financial position. We believe that our compensation program has helped us both recruit and retain superior executive talent to continue to build an organization capable of executing on our mission to become a leading research-based development stage biopharmaceutical company.
COMPENSATION DETERMINATION PROCESS
ROLE OF ORGANIZATION AND COMPENSATION COMMITTEE
The Organization and Compensation Committee is responsible for establishing the compensation programs of the Company’s CEO and other NEOs. The Committee also administers the Company’s equity-based and performance-based compensation plans, including plans under which restricted stock units and options are awarded. Accordingly, it is responsible for reviewing cash and equity incentives payable to executives and has the authority to grant restricted shares of Company Common Stockstock units and options to purchase shares of the Company’s Common Stock to all participants under the Company’s equity award plans, and to determine all terms and conditions of such awards.
ROLE OF MANAGEMENT
To aid the Organization and Compensation Committee in its responsibilities, our Chief Executive Officer provides the committee with recommendations relating to the performance and achievements of each of the NEOs (other than himself). The Organization and Compensation Committee gives considerable weight to the Chief Executive Officer’s performance evaluations of the other NEOs because he has direct knowledge of the criticality of their work, performance and contributions. The committee does not consult with any other executive officers with regard to its decisions. The Chief Executive Officer does not participate in the committee’s deliberations or decisions regarding his own compensation.
ROLE OF COMPENSATION CONSULTANT
In 2022,2023 the Organization and Compensation Committee continued to retain the services of Aon plc as its independent executive compensation consultant due to its extensive analytical and compensation expertise in our industry. In this capacity, Aon has advised the Organization and Compensation Committee on compensation matters related to the executive and director compensation programs including:
executive and director market pay analysis;
reviewing employee equity award framework;
reviewing and, when appropriate, suggesting changes to the compensation peer group;
development and refinement of executive pay programs and governance practices; and
assistance in preparing thisreviewing the Compensation Discussion and Analysis and other proxy statement disclosures.
Additionally, the Company annually participates in Aon’s compensation surveys. The Organization and Compensation Committee has the sole authority to engage and terminate Aon’s services, as well as to approve its compensation. Aon makes recommendations to the committee but has no authority to make compensation decisions on behalf of the committee or the Company. Aon reported to the Organization and Compensation Committee and had direct access to the chairperson and the other members of the committee. Beyond data and advice related to executive and director compensation matters and equity plan design and assistance with the preparation of proxy statement disclosures, Aon did not provide any other consulting services to us in 2022.2023.
The Organization and Compensation Committee conducted a specific review of its relationship with Aon in 20222023 and determined that Aon’s work did not raise any conflicts of interest. Aon’s work has conformed to the independence factors and guidance provided by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, the SEC and Nasdaq.
USE OF MARKET DATA AND PEER GROUP ANALYSIS
We regularly review the compensation practices of our peers in response to the fast-moving nature of the biotechnology industry, including merger and acquisition activity, and changes in product pipeline and business stage. As a result of the Company having a combination of multiple drug candidates in diverse therapeutic areas,
44
a mix of wholly-owned and partnered drug candidates, a technology platform with the potential to enable multiple drug candidates in future years, and a legacy proprietary manufacturing operation, it is very challenging to identify truly comparable companies. AsHistorically, we conduct an annual peer group review in June of each year. In June 2022, as a result of theour 2022 Restructuring Plan,reorganization and ongoing strategic changes to our business, the Organization and Compensation Committee, with input from our independent compensation consultant, Aon, decided to retain the peer group selected in June 2021 for the remainder of 2022 and the beginning of 2023.
In June 2023, we engaged Aon to focus on retention of key executives who were critical to the successconduct a review and assessment of our 2022 Restructuring Plan. The Organizationpeer group. Previously, we had selected our peer group companies by considering companies in a comparable sector and Compensation Committee also believedstage with market capitalizations between 0.5x to 3.0x of our market capitalization at the time of review. However, a company’s market capitalization can at times be misaligned with market expectations. We believe that, the retentiondue to current market conditions and in light of the 2021fact that our market valuation has been trading below our cash value, the use of market capitalization as the primary criteria for selecting our peer group was appropriate as no merit adjustments to executive base compensation were made in 2022. Therefore, no changes to the peer group, which was established in 2021, were made in 2022. In determiningwould not best capture an appropriate peer group that is comparable to our committee workedcompany. In consultation with Aon, we calculated and examined the ratio of market capitalization to identify potentialcash value within peers utilizingto determine a median “cash value” multiple, which we applied to our cash value at the following criteriatime of review to derive an implied valuation of the Company. We then considered for its reviewour peer group, companies in June 2021:a comparable sector and stage with market capitalizations between 0.5x to 3.0x of our implied valuation and further selected our peer group based on comparable financial profile, business and product focus, and development stage.
As a result, the following peer group was retainedused for evaluating 20222023 - 2024 compensation decisions:
| 4D Molecular Therapeutics | | | BioAtla | | | Kodiak Sciences |
| Alector | | | | | ||
| Allakos | | | | | Olema Pharmaceuticals | |
| Alpine Immune Sciences | | | Erasca | | | ORIC Pharmaceuticals |
| ALX Oncology | | | Gossamer bio | | | RAPT Therapeutics. |
| Annexon | | | IGM Biosciences | | | |
| Aura Biosciences | | | Kezar Life Sciences | | |
| * | Was included in the 2021 and 2022 peer group. |
As compared to the 2021 and 2022 peer group, our 2023 peer group includes all new companies, except for bluebird bio, Inc. (marked with an asterisk in the above table).
Given that our peer group companies have different market capitalizations than the Company, the Organization and Compensation Committee also reviews equity and total direct compensation data for our executives against the compensation for similarly situated executives at peer companies contained in surveys. Although the Organization and Compensation Committee reviewed and discussed the compensation data for the peer group companies to help inform executive compensation decisions, it does not set compensation at any specific level or percentile based solely on the peer group data. The peer group data and general industry compensation survey data is used only as one reference point considered in making compensation decisions. Other factors considered include an assessment of individual and company performance, competitive market practices, the number of unvested stock options held by the executive and average exercise price (i.e., the retention value) of these options, the number of unvested RSUs, and PSUs, the individual’s overall contributions, and stockholder dilution. However, we do not use a formula or assign a particular weight to any one factor in determining cash and equity award levels.
DESIGN AND ELEMENTS OF OUR COMPENSATION PROGRAM
In 2022,2023, the executive compensation structure featured three primary elements:
base salary;
short-term cash incentives, based on the Company’s achievement of pre-established corporate performance objectives as well as individual performance; and
long-term incentive,incentives, awarded as a mix of time- and performance-based RSUs and option grants.
45
Compensation Committee consulted with Aon, its independent executive compensation consultant, to design a compensation program that provides appropriate incentives and opportunities to the executives, aligns the vesting and performance milestones for equity awards with the Company’s revised strategic plan, reflects the Company’s current research and clinical developments, and remains market-aligned with industry peer groups, among others. Each element of our compensation program is discussed further below.
BASE SALARY
Base salary is the initial building block of compensation for the NEOs because it provides the executives with a specified basic level of cash compensation, which we believe is important to attract and retain highly skilled and experienced executives. The Organization and Compensation Committee determines base salaries by considering competitive pay practices, cost of labor and compensation trends, individual performance and promotions, level and scope of responsibility, experience and internal pay equity. However, the Organization and Compensation Committee does not use a formula or assign a particular weight to any one factor. Rather, the determination of base salary levels is subjective, and base salaries are set at levels that we believe to be reasonably competitive. Based on these factors, the Organization and Compensation Committee decided not to change executive base salaries for 2021 from 2020 levels. In 2021, the Organization and Compensation Committee made modest increases to the 2022 executive base salaries to reflect the increased executive responsibilities in connection with the growth of the Company in preparation for commercial readiness. In July 2022,readiness at the time. The Organization and Compensation Committee reviewedhas since not awarded any merit-based increases to our NEO’s base compensation for the base salaries of the NEOs for 2023 and determined that no merit increases would be made to the base salaries of the NEOs for 2023.last three years since 2021. Ms. Thomsen, who previously served as our Senior Vice President, Financeformer Chief Financial Officer and Chief Accounting Officer, was appointed our Chief Financial Officer as of July 1, 2022.and Mr. Wilson, who previously served as our General Counsel, was appointed our Chief Legal Officer, as of July 1, 2022. In connection with their new appointments, Ms. Thomsen and Mr. Wilson received a promotion-based increase to their respective base salaries to reflect their increased duties and responsibilities, which was made effective as of July 1, 2022.
Name | | | 2021 | | | 2022 | | | 2023 | | | 2022 to 2023 % Increase |
Howard W. Robin | | | $1,053,000 | | | $1,084,590 | | | $1,084,590 | | | 0% |
Jillian B. Thomsen(1) | | | — | | | $494,000 | | | $550,000 | | | 11.3% |
Mark A. Wilson(2) | | | $500,000 | | | $515,000 | | | $540,000 | | | 4.8% |
Jonathan Zalevsky, Ph.D. | | | $683,000 | | | $703,490 | | | $703,490 | | | 0% |
Gil M. Labrucherie(3) | | | $788,000 | | | $811,640 | | | — | | | — |
John Northcott(4) | | | $675,000 | | | $695,250 | | | — | | | — |
| Name | | | 2022 | | | 2023 | | | 2024 | | | 2023 to 2024 % Increase |
| Howard W. Robin | | | $1,084,590 | | | $1,084,590 | | | $1,084,590 | | | 0% |
Sandra Gardiner(1) | | | — | | | — | | | — | | | — |
| Mark A. Wilson | | | $540,000(3) | | | $540,000 | | | $540,000 | | | 0% |
| Jonathan Zalevsky, Ph.D. | | | $703,490 | | | $703,490 | | | $703,490 | | | 0% |
Jillian B. Thomsen(2) | | | $550,000(4) | | | $550,000 | | | — | | | — |
| (1) | Ms. Gardiner was appointed our Interim Chief Financial Officer as of April 17, 2023 and provides services as Interim Chief Financial Officer as an outside consultant pursuant to a consulting agreement between the Company and FLG Partners and is not employed or directly compensated by us, as further described under “Certain Relationships and Related Party Transactions.” |
| (2) | Ms. Thomsen served as our Chief Financial Officer and Chief Accounting Officer from July 1, 2022 to April 17, 2023, and her employment with the Company ended on June 16, 2023. Additional information regarding the compensation Ms. Thomsen received in 2023 is reported in the Summary Compensation Table—Fiscal 2021-2023. |
| (3) | Mr. Wilson previously served as our General Counsel. Mr. Wilson was appointed our Chief Legal Officer as of July 1, 2022 and received a promotion-based increase to his salary that was made effective as of July 1, 2022. His 2022 base salary prior to July 1, 2022 was $515,000. |
| (4) | Ms. Thomsen previously served as our Senior Vice President, Finance and Chief Accounting Officer. Ms. Thomsen was appointed our Chief Financial Officer and Chief Accounting Officer as of July 1, |
SHORT-TERM INCENTIVES
We believe that our short-term incentive compensation program (“Incentive Compensation Plan”) rewards the achievement of important short-term objectives that advance us toward our long-term strategic objectives. Our Incentive Compensation Plan applies to all employees and all executive officers other than our Chief Executive Officer, Mr. Robin, who is subject to his own separate annual performance-based bonus compensation arrangement with a combination of corporate and personal objectives established and evaluated by the Organization and Compensation Committee pursuant to Mr. Robin’s amended and restated offer letter effective as of December 1, 2008. As in 2021 and 2022, Mr. Robin’s bonus arrangement for 20222023 was based on the same corporate objectives that we established under the Incentive Compensation Plan to greater align Mr. Robin’s short-term incentive compensation with the achievement of important short-term objectives of the Company. Consistent with
our compensation philosophy of paying for performance and maintaining a flexible approach, we use the Incentive Compensation Plan to incentivize the NEOs to achieve important corporate goals while at the same time encouraging and rewarding excellent individual performance by recognizing and rewarding differences in performance between individual executives.
46
2023 and 20212024 Target Annual Incentive Opportunities
The NEOs were each assigned a target annual incentive for 20222023 ranging from 60% to 100% of base salary. The target annual incentive opportunities are determined based on each NEO’s experience, scope of responsibilities, and potential impact on the Company’s performance and approved by the Organization and Compensation Committee. The table below shows the target annual incentive assigned by us to each NEO as of December 2022 both as a dollar amount and as a percentage of base salary.salary for 2023 and 2024. Target annual incentives for each NEO remained the same for 2024 as they did for 2023.
As further discussed below, each NEO’s (excluding Mr. Robin’s) annual bonus is determined based on a combination of the corporate performance rating and individual performance. In 2021, we began to directly align Mr. Robin’s, the CEO’sCEO, annual bonus award with the Company’s corporate performance rating. As a result,so that Mr. Robin’s annual bonus is determined based solely on the Company’s corporate performance rating. Because Mr. Robin’s annual bonus is dependent solely on the performance of the Company, his target annual incentives forsince 2021 and 2022 werehave been set at 100%.
| | | 2021 Target Annual Incentives | | | 2022 Target Annual Incentives | |
Name | | | (% of Base Salary) | | | (% of Base Salary) |
Howard W. Robin | | | 100% | | | 100% |
Jillian B. Thomsen(1) | | | — | | | 60% |
Mark A. Wilson | | | 50% | | | 60% |
Jonathan Zalevsky, Ph.D. | | | 60% | | | 60% |
| | | 2023 Target Annual Incentives | | | 2024 Target Annual Incentives | |
| Name | | | (% of Base Salary) | | | (% of Base Salary) |
| Howard W. Robin | | | 100% | | | 100% |
Sandra Gardiner(1) | | | — | | | — |
| Mark A. Wilson | | | 60% | | | 60% |
| Jonathan Zalevsky, Ph.D. | | | 60% | | | 60% |
Jillian B. Thomsen(2) | | | 60% | | | — |
| (1) | Ms. Gardiner does not participate in our Incentive Compensation Plan. |
| (2) | Ms. Thomsen |
Company Performance Objectives
The board of directors established at the beginning of 2022annually establishes a small number of important annual corporate goals that include clinical development, research, commercial, manufacturing, organizational and financial goals which we believe are essential to building long-term stockholder value. These goals are then used in our Incentive Compensation Plan to assess annual corporate performance. The relative weightings of these corporate goals are based upon our assessment of the importance of each goal in creating long-term value for the Company and our stockholders. If we achieve the target level of performance for all of the stated goals, the overall corporate performance rating should be approximately 100%. We endeavor to select corporate goals that, if met by management, represent significant levels of annual achievement, although we believe the long-term nature of our drug development business does not lend itself to over-weighting the importance of annual goals.
Following the conclusion of the annual performance period in December of each year, the level of achievement for each corporate goal is assessed by the board of directors. The board of directors determines whether each corporate goal has been met, exceeded, or not satisfied. In addition, in assessing corporate performance, the determination of corporate performance may be adjusted upward or downward as deemed appropriate to factor in other significant corporate events, either negative or positive, that occurred during the performance period, but were not reflected in the corporate goals previously set by the board of directors. After taking into account the level of attainment of each corporate goal and such other corporate performance factors as the board of directors may determine appropriate in reviewing performance for a particular year, the board of directors assigns an overall corporate performance rating for the year, which may range from 0% to 200%. The Organization and Compensation Committee then confirms the corporate performance rating for purposes of the Incentive Compensation Plan. Historically, theThe total available bonus pool under the Incentive Compensation Plan is determined by multiplying the corporate performance rating by the aggregate target bonus of all eligible participants which includes nearly all of the Company’s full-time employees. The aggregate of all individual bonuses awarded under the plan cannot exceed the total available bonus pool so that the total cost of bonuses ultimately reflects our assessment of overall corporate performance and is not inflated by the sum of individual performance ratings. Mr. Robin does not participate in the final selection of the corporate goals or determination of the corporate performance rating.
After the corporate performance rating is determined, the individual performance of each NEO is reviewed by the Organization and Compensation Committee in consultation with. Mr. Robin (other than with respect to his own performance) in order to determine the appropriate individual performance percentage rating to be assigned to the executive for the performance period. Mr. Robin’s individual performance is separately reviewed by the Organization and Compensation Committee. Each NEO’s (excluding Mr. Robin’s) actual annual bonus is based on a combination of the corporate performance rating and individual performance. The Incentive Compensation Plan does not provide for a specific allocation or weighting between corporate and individual performance. The actual annual bonus awarded for each NEO (excluding Mr. Robin) is solely determined by the Organization and Compensation Committee based on criteria that includes an assessment of individual performance (as measured by Mr. Robin’s evaluation of the performance of each NEO) and company performance, and the maximum payout for each NEO could be up to 200% of his or her cash target annual incentive (or, by the same token, an individual executive’s award could be reduced to 0% based on individual performance regardless of the corporate performance rating). Mr. Robin’s annual bonus award is directly aligned and based on the corporate performance rating that is recommended by the Organization and Compensation Committee and approved by the board of directors, which may range from 0% to 200%. Given the dynamic nature of our business, new priorities continually emerge such that the Organization and Compensation Committee wishes to retain the flexibility to tie a varying portion of annual incentive payouts to the individual achievement of a range of objectives.
In January 2022,2023, the board of directors formally approved the 20222023 corporate objectives (the “2022“2023 Corporate Objectives”) set forth below. Several of the corporate objectives were connected to the outcome of the Company’s bempegaldesleukin clinical trials. As a result of the Company’s termination of the bempegaldesleukin program and the 2022 Restructuring Plan, the Company’s 2022 corporate performance rating was not considered in determining the annual bonuses awarded to the Company’s eligible non-executive employees under the Incentive Compensation Plan. However, the board of directors determined that the Company’s 2022 corporate performance rating, as measured against the 2022The 2023 Corporate Objectives, would continue to be used to calculate the annual bonus awards for each NEO, including Mr. Robin. We believe this aligns with our pay for performance philosophy and provides an assessment of management’s performance that is reflective of the Company’s overall annual performance.
| | Category | | | Weight | | | Objective | | | Results(1) | | | Category Raw Score | | | Category Weighted Score | |
| | Bempegaldesleukin Regulatory | | | 35% | | | Acceptance of regulatory filings for bempegaldesleukin in melanoma | | | (c) | | | 0.0(2) | | | 0.0 | |
| | Acceptance of regulatory filings for bempegaldesleukin in renal cell carcinoma or bladder cancer | | | (c) | | ||||||||||||
| | Complete regulatory inspections in connection with the bempegaldesleukin program | | | (c) | | ||||||||||||
| | Commercial Readiness | | | 10% | | | Various objectives that achieve bempegaldesleukin commercial readiness in the U.S. | | | (b) | | | 0.5 – 0.7(3) | | | 0.05 – 0.07 | |
| | Various objectives that achieve bempegaldesleukin commercial readiness in the E.U. | | | (b) | |
| | Category | | | Weight | | | Objective | | | Results(1) | | | Category Raw Score | | | Category Weighted Score | |
| | Corporate | | | 20% | | | Secure rights to rezpegaldesleukin | | | (a)(2) | | | 1 | | | .20 | |
| | Complete 2023 corporate restructuring | | | (a)(3) | | ||||||||||||
| | Achieve year-end cash target | | | (a)(4) | | ||||||||||||
| | Development | | | 25% | | | Advance clinical study of rezpegaldesleukin in atopic dermatitis | | | (a)(5) | | | 1 | | | .25 | |
| | Advance clinical study of rezpegaldesleukin in other indications | | | (a)(6) | | ||||||||||||
| | Advance clinical studies of NKTR-255 in oncology | | | (a)(7) | | ||||||||||||
| | Research | | | 15% | | | Engage contract research organizations to continue research development | | | (a)(8) | | | 1 | | | 0.15 | |
| | Advance research pipeline candidates | | | (a)(9) | | ||||||||||||
| | Manufacturing/ Process Development | | | 15% | | | Ensure near term supply of rezpegaldesleukin | | | (a)(10) | | | .87 - .93 | | | 0.13-0.14 | |
| | Support toxicology studies for other pipeline candidates | | | (b)(11) | | ||||||||||||
| | Business Development | | | 25% | | | Various objectives related to business development | | | (a)(12) | | | 0.64-0.76 | | | 0.16-0.19 | |
| | Reduce real estate obligations | | | (b)(13) | | ||||||||||||
| | Establish a global collaboration for NTKR-255 | | | (b)(14) | | ||||||||||||
| | | | | | | | Corporate Performance Rating Range | | | 0.89-0.93 | | ||||||
| | Category | | | Weight | | | Objective | | | Results(1) | | | Category Raw Score | | | Category Weighted Score | |
| | Clinical Development | | | 25% | | | Advance enrollment for a registrational study of bempegaldesleukin in lung cancer | | | (b)(4) | | | 0.72 – 0.80 | | | 0.18 – 0.20 | |
| | Advance enrollment for a registrational study of bempegaldesleukin in head and neck cancer | | | (c)(2) | | ||||||||||||
| | Advance enrollment for a registrational study of bempegaldesleukin in melanoma | | | (a)(5) | | ||||||||||||
| | Advance clinical studies of NKTR-255 | | | (a)(6) | | ||||||||||||
| | Research | | | 10% | | | Advance a new drug development candidate to support an IND filing in 2023 | | | (a)(7) | | | 1.00 | | | 0.10 | |
| | Identify a new drug development candidate for next development program | | | (a)(8) | | ||||||||||||
| | Manufacturing | | | 10% | | | Develop commercial product and manufacturing process for NKTR-255 | | | (b) | | | 0.6 – 0.7(9) | | | 0.06 – 0.07 | |
| | Develop toxicology supplies for NKTR-288 | | | (b) | | ||||||||||||
| | Corporate and Business Development | | | 10% | | | Various objectives related to corporate organization and employee development goals | | | (a)(10) | | | 1.1 – 1.3 | | | 0.11 – 0.13 | |
| | Establish new strategic collaborations | | | (a)(11) | | ||||||||||||
| | | | | Corporate Performance Rating Range | | | 0.50 – 0.57 | | |||||||||
| (1) | (a) met or exceeded; (b) partially |
| (2) | Objective was |
| (3) |
| (4) | Objective was |
48
| (5) | Objective was met. |
| (6) | Objective was met. |
| (7) | Objective was met. |
| (8) | Objective was met. |
| (9) |
| (10) | Objective was met. |
| (11) | Partially met. Prioritized toxicology studies for NKTR-1065 drug candidate. |
| (12) | Objective was |
| (13) | Partially met. Continued efforts to sublease certain portions of our unused office facilities. |
| (14) | Partially met Executed a new clinical study collaboration with AbelZeta to study NKTR-255 in combination with AbelZeta’s TIL therapy in NSCLC patients. |
The weighting of these objectives is a reflectionreflective of our long-term focus as a research-based development stage biopharmaceutical company. As such, in establishingcompany as well as our shorter-term business priorities. Our board of directors carefully considers the 2022 Corporate Objectives, we overweighted the percentage of achievement measurement for objectives related to development progress and outcomes, particularly for those associated with the bempegaldesleukin program, our lead program at the time. We believe thisappropriate mix and weighting of corporate goals places emphasis on milestones that were importantwill help motivate the performance of our executives as well as to build long-term sustainable foundation ofgrowth for our business at the time.business. A corporate performance rating in excess of 100% can only be achieved if the board of directors determines that the goal achievement for one or many of the goals substantially and qualitatively exceeded the target metrics, or the Organization and Compensation Committee uses its discretion to factor in other significantly positive corporate events that occurred during the performance period. The maximum potential corporate performance rating is 200%.
Actual Annual Incentives Earned for 20222023
Management prepared a report on the status of achievement of the 20222023 Corporate Objectives that was reviewed by the Organization and Compensation Committee in December 2022.2023. The Organization and Compensation Committee determined that sixten of the corporate goals identified above were met. Each of the goals that was “met” had to achieve objective and specified measurement criteria established by the board of directors in the beginning of the year. If the measurement criteria for an objective was not fully met, partial credit was given depending on the level of achievement. Although we and BMS terminated the bempegaldesleukin program in April 2022, the Organization and Compensation Committee recommended that partial credit should also be acknowledged for the development progress achieved in 2022. As noted in the footnotes to the table, we have highlighted some of the specific achievements made within each objective category but becausethat was evaluated by the board of directors in determining whether such Corporate Objective was met. Due to the sensitivity and proprietary nature of our business and certain confidentiality obligations, we cannot disclosespecify all of the achievements made by the Company in furtherfurtherance of its 2022our 2023 Corporate Objectives. Using a raw score range for the achievement of the objectives within each category, and then calculating the weighted score for each category, the 20222023 corporate performance rating was between the range of 50%89% to 57%93%.
We presented new responder data for rezpegaldesleukin in a late-breaking news oral presentation at the 2023 European Academy of the bempegaldesleukin program. As of September 2022,Dermatology and Venereology Congress that showed dose-dependent efficacy across all endpoints for patients have ended bempegaldesleukin treatment, have been transitioned to standard of care treatment, or enrolledwith moderate-to-severe atopic dermatitis who were treated with rezpegaldesleukin in the Post Trial Access Program.Phase 1b study. The data showed rapid onset of action for rezpegaldesleukin and continued benefit for 36 weeks after 12-week treatment period.
49
We created new development opportunities for rezpegaldesleukin within our runway budget.
In December 2022,2023, we initiatednegotiated the repurchase of shares of our common stock that were previously purchased by Bristol-Myers Squibb Company. The repurchase was subsequently completed in February 2024 for a Nektar-sponsored Phase 2/3 study (currentlypurchase price that was discounted from the 30-day volume-weighted average price per share of our common stock. The reduction in our outstanding shares will be reflected fully in the Phase 2 portion) to evaluate NKTR-255 following CD19 CAR-T cell therapy in patients with large B-cell lymphoma.
In view of these achievements, andas well as the Company’s efforts to successfully launchCompany having met or exceeded a new strategic plan, and ability to retain key institutional knowledge followingsubstantial number of the 2022 Restructuring Plan2023 Corporate Objectives, the Organization and Compensation Committee recommended to the board of directors (which the board of directors subsequently approved) a corporate performance achievement of 60%.95% for 2023.
The following table shows some of the highlights of each NEO’s performance in 2022.2023:
Name | | | Individual Performance Highlights |
| Howard W. Robin | | | • Focused the Company on advancing the development of rezpegaldesleukin. |
| | | ||
| Mark Wilson | | | • Led the strategy for our complaint filed against Lilly and successfully defended the Company against other litigation exposure. |
| | | ||
| Jonathan Zalevsky, Ph.D. | | | • Led the • |
The table below includes the actual 20222023 bonuses, including as a percentage of the target opportunity, that we awarded the NEOs for 2022.2023. Mr. Robin’s awarded annual incentive wasis directly aligned to the Company’s corporate performance rating, of 60%which was 95%. In determining the annual incentive for each of our other NEOs, our Organization and Compensation Committee considers the Company’s corporate performance rating, as well as each NEO’s individual performance and accomplishments highlighted above. For 2022,2023, each other NEO’s annual incentive was also directly aligned with the Company’s corporate performance rating of 60%95%. Ms. Gardiner, our Interim Chief Financial Officer, provides services as an outside consultant and is not employed or directly compensated by us, as further described under “Certain Relationships and Related Party Transactions.” Ms. Gardiner does not participate in our Incentive Compensation Plan and did not receive any bonus payment in 2023.
| | | 2022 Target Annual Incentives | | | 2022 Earned Annual Incentives | |||||||
Name | | | (% of Base Salary) | | | ($) | | | (% of Target Bonus) | | | ($) |
Howard W. Robin | | | 100% | | | $1,084,590 | | | 60% | | | $650,754 |
Jillian B. Thomsen | | | 60% | | | $330,000 | | | 60% | | | $198,000 |
Mark A. Wilson | | | 60% | | | $324,000 | | | 60% | | | $194,400 |
Jonathan Zalevsky, Ph.D. | | | 60% | | | $422,094 | | | 60% | | | $253,256 |
| Name | | | 2023 Target Annual Incentives | | | 2023 Earned Annual Incentives | ||||||
| | (% of Base Salary) | | | ($) | | | (% of Target Bonus) | | | ($) | ||
| Howard W. Robin | | | 100% | | | $1,084,590 | | | 95% | | | $1,030,361 |
| Mark A. Wilson | | | 60% | | | $324,000 | | | 95% | | | $307,800 |
| Jonathan Zalevsky, Ph.D. | | | 60% | | | $422,094 | | | 95% | | | $400,989 |
Jillian B. Thomsen(1) | | | 60% | | | $330,000 | | | 0% | | | $0 |
| (1) | Ms. Thomsen served as our Chief Financial Officer and Chief Accounting Officer from July 1, 2022 to April 17, 2023, and her employment with the Company terminated on June 16, 2023. As discussed further below, she did not receive any annual incentive compensation for 2023. |
LONG-TERM INCENTIVES
In accordance with our objective of aligning executive compensation with our stockholders’ interests, an important component of our executive compensation program are long-term incentive opportunities. Our current long-term incentive program for the NEOs generally consists of annual awards of equity compensation that are subject to multi-year time-based vesting schedules as well as performance-based vesting schedules. InSince 2018,
50
we adoptedhave used a value-based approach for sizing equity awards, consistent with market practices. In determining the grant levels for equity awards, we generally consider a number of factors including an assessment of individual performance, competitive market practices, the number of unvested RSUs and stock options held by the executive and average exercise price (i.e., the retention value) of these stock options, the individual’s overall contributions, and stockholder dilution. However, we do not use a formula or assign a particular weight to any one factor in determining equity award levels. Rather, the determination of equity grant levels is subjective, and the Organization and Compensation Committee awards equity grants at levels it believes in its judgment are reasonably competitive and consistent with our philosophy that a substantial portion of our executives’ compensation should be performance-based and help to further link the interests of our executives with those of our stockholders, as well as to provide a retention incentive for the executive as well as an additional incentive to help create value for our stockholders.executive.
We historically annually review our executive compensation and grant equity grants in December of each year. In 2022, in connection with the 2022 Restructuring Plan andour restructuring efforts, the Organization and Compensation Committee’sCommittee conducted its executive compensation review in July 2022 the NEOs received the following annual equity grants in August 2022. These equity grants effectivelyand “pulled forward” the timing of any annual equity grants that would have otherwise been considered in December 2022. No additional equity grants were made to our executives in 2022, which resulted in an 18-month gap in grant timing between July 2022 and enabledDecember 2023. In designing the 2023 equity grants, the Organization and Compensation Committee, in consultation with Aon, evaluated the holding power of our executive’s existing equity, internal pay equity, and other factors. The Organization and Compensation Committee also noted that, due to the Company’s current low stock price, all existing options held by our executives are “underwater” or trading below their strike price, and that the value of our executives’ unvested equity, based on market data, is below the value of potential new equity grants that our executives could receive at new positions at another company, which could contribute to focus on our restructuring efforts and executing the Company’s new strategic plan.retention risk. We also recognize that pharmaceutical research and development require sustained effort over many years. The equity grants cover multi-year time framesyears and short term fluctuations are designednot uncommon. In order to incentivizebalance the Company’s interests to motivate and incentive our executives to perform highly and achieve important researchbusiness objectives, to retain talented and business development objectivesexperienced executives and to maximize stockholder value, the Organization and Compensation Committee recommended to the board of directors (which the board of directors subsequently approved) that will contributethe equity grants to our long-term success. No additionalexecutives in 2023 consist of stock options only, with 50% subject to time-based vesting and 50% subject to performance-based. The following equity grants were madeawarded to our NEOs in December 20222023. Ms. Gardiner did not receive any equity grants. Ms. Thomsen, our former Chief Financial Officer and Chief Accounting Officer, did not receive any equity grants in 2023 because her employment with the next executive compensation review is planned to occur in DecemberCompany ended on June 16, 2023.
| | | Time-based Awards (50%) | | | Performance-based Awards (50%) | |||||||
Name | | | Stock Options (#) | | | RSUs (#) | | | Stock Options (#) | | | RSUs (#) |
Howard W. Robin | | | 821,250 | | | 410,625 | | | 821,250 | | | 410,625 |
Jillian B. Thomsen | | | 331,875 | | | 165,938 | | | 331,875 | | | 165,938 |
Mark A. Wilson | | | 331,875 | | | 165,938 | | | 331,875 | | | 165,938 |
Jonathan Zalevsky, Ph.D. | | | 270,000 | | | 135,000 | | | 270,000 | | | 135,000 |
| | | Stock Options (Number of Shares(#)) | ||||
| Name | | | Time-based Awards (#) (50%) | | | Performance-based Awards (#) (50%) |
| Howard W. Robin | | | 1,300,000 | | | 1,300,000 |
| Mark A. Wilson | | | 325,000 | | | 325,000 |
| Jonathan Zalevsky, Ph.D. | | | 250,000 | | | 250,000 |
Time-Based Stock Options and RSUs
The Organization and Compensation Committee believes that the granting of time-based RSUs and stock options are an important component of the equity vehicle mix. RSUs provide a strong retention element to the compensation program, while stockStock options represent a direct alignment of executive interests with those of shareholders.stockholders and motivate executives to achieve value-enhancing objectives for the Company. Stock options also create strong retention incentives. In 2022,2023, 50% of the equity awards granted to our NEOs were time-based equity awards. The time-based stock options vest monthly over three years, and the time-based RSUS vest quarterly over three years, subject to the executive’s continued service through the vesting date.
Performance-Based Equity
In 2022,2023, 50% of the equity awardsstock options granted to our NEOs were performance-based equity awards that are subject to both time-based and performance-based vesting. Performance-based options vest only upon achievement of measurable performance goals tied to our business strategy, which are set forth below. They help
51
create an ownership culture, encourage individual contribution and provide a strong link between executive performance and the Company’s long-term value. In order to vest, within three years of grant of the performance-based stock options, and RSUs the Company must achieve at least twoone of the following performance milestones:
The Organization and Compensation Committee believed these performance criteria would be challenging to achieve and if achieved, would help create long-term stockholder value. These performance milestones were set to align the interest of our executives with those of the Company’s newrenewed strategic goals.focus on advancing rezpegaldesleukin.
In responsethe past, our executive equity compensation package has included additional components and vehicles such as time-based and performance-based RSUs and performance-based RSUs tied to relative total shareholder return performance. Given the business changes and current stage and size of our Company’s growthcompany, we believe that granting stock options provides the appropriate incentives for our executives to continue performing highly while maximizing stockholder and stockholder feedback, in 2020 thecompany value. The Organization and Compensation Committee introduced a new component to our equity compensation in order to increase the percentage of equity grants to our senior executives that were subject to performance-based conditions and to reflect, at the time, the Company’s maturation into a biopharmaceutical company with a potential commercialized drug. In 2020 and 2021, our senior executives were granted new performance-based RSUs (“TSR RSUs”) that vest between 0% and 200% of the target award based on relative total shareholder return (TSR) performance as measured against the Nasdaq Biotechnology index over a two-year performance period. The TSR RSUs were granted in order to align the interests of our executives more closely with shareholder return. For 2022, the Organization and Compensation Committee did not grant any relative TSR RSUs as part of the executive compensation structure. We believe that the long-term success of the Company depends on our ability to develop drug candidates that can demonstrate positive clinical results, receive regulatory approvals and achieve commercial success. Following our 2022 restructuring and given the current stage of our Company, we believe that our executives should be incentivized to prioritize our core research programs, to advance our drug candidates through clinical trials and to form strategic collaborations, which will ultimately lead to the creation of shareholder value and appreciation in value of our stock. To that end, the Organization and Compensation Committee determined that the granting of performance-based stock options and RSUs tied to the achievement of critical objective, development-based performance milestones instead of short-term stock value, would be more effective to align the interests of our NEOs with the long-term interests and goals of the Company and our stockholders. The Organization and Compensation will continue to evaluate our executive compensation program in light of the Company’s changing business developments and it may grant TSR RSUs or other related awards tied to short-term stock value in the future when such approaches are believed to be in the best interests of the Company and its shareholders.
The performance criteria associated with the performance-based stock options and RSUs granted to our senior executives and NEOs in each of 2017, 2018 and 2019 have been met. The 2017 performance-based stock options and RSUs granted on December 15, 2017 began vesting after the FDA’s July 2018, acceptance for review of the Company’s NDA for NKTR-181. As of December 31, 2022, all of the 2017 performance-based stock options and RSUs have vested in full. The 2018 performance-based stock options and RSUs granted on December 14, 2018 began vesting after the Organization and Compensation Committee determined on March 13, 2022, that all required performance criteria had been achieved, on March 13, 2022, which included the achievement of the first patient dosed in four registration trials for bempegaldesleukin run by the Company and the filing and acceptance of two investigational new drug applications (IND) with the FDA for drug candidates wholly-owned by the Company. As of December 31, 2022, all of the 2018 performance-based stock options and RSUs have vested in full. The 2019 performance-based stock options and RSUs granted on December 12, 2019 began
vesting after the Organization and Compensation Committee determined on September 15, 2021 that the required performance criteria had been achieved, on September 15, 2021, with (i) the FDA’s acceptances of an IND for NKTR-255 in solid tumors in August 2020 and an IND for bempegaldesleukin for COVID-19 treatment, and (ii) the execution of a co-development agreement with SFJ Pharmaceuticals and a clinical trial collaboration and supply agreement with Merck to support the development of bempegaldesleukin, as well as an oncology clinical collaboration with Merck KGaA, Darmstadt, Germany and Pfizer Inc. to evaluate NKTR-255 in combination with avelumab in patients with locally advanced or metastatic urothelial carcinoma in the Phase II JAVELIN Bladder Medley study, whereupon approximately 22/48th of the performance-based options vested, followed by continued monthly pro-rata vesting of the remainder until December 12, 2023. As of December 31, 2022,2023, all of the 2019 performance-based options and RSUs have vested in full.
2020 - 2022 Grants of Performance Based-Equity
The performance criteria associated with each of the performance stock options and RSUs granted to our senior executives and NEOs in 2020 have not been met and thus, vesting of these performance-based stock options and RSUs has not occurred. If the performance criteria associated with these performance stock options and RSUs are not satisfied within five years of grant, the equity awards will be forfeited.cancelled.
52
total shareholder return percentile rank(TSR) performance as measured against the Nasdaq Biotechnology Index at the end of theindex over a two-year performance period. The performance period for the 2020 TSR RSUs was measured from December 18, 2020 to December 31, 2022 (the “2020 TSR RSU Performance Period”), the grant recipients could vest between 0% and 200%, the performance multiplier, of the target TSR RSUs granted.. Following the 2020 TSR RSU Performance Period, the Organization and Compensation Committee engaged Aon to independently determine the Company’s TSR percentile rank within the Nasdaq Biotechnology Index. Aon determined that the Company’s performance measured at the 15th percentile, which pursuant to the terms of the TSR RSUs grant, corresponds to a performance multiplier of 0%. Therefore, the Organization and Compensation Committee determined that none of the 2020 TSR RSUs that were granted to our senior executives and NEOs would vest. Accordingly, all 2020 TSR RSUs granted will not vest.
In 2021, approximately 37% of the annual equity awards granted to our NEOs were performance-based stock options and RSUs that would vest only upon the achievement of certain performance criteria. Our NEOs were granted certain performance-based stock options and RSUs (the “2021 Performance Grants”) that would only vest if the Company achieved the first commercial sale to a third party of bempegaldesleukin intended for use by end-user costumer within five years of the award grant date. In 2022, we and BMS discontinued all clinical development activities of bempegaldesleukin. Although the performance period for the 2021 Performance Grants has not ended yet, due to the termination of our bempegaldesleukin development program, achievement of the performance criteria for the 2021 Performance Grants is no longer realistically feasible.
As discussed above, our NEOs were also granted TSR RSUs in 2021. The performance period for the 2021 TSR RSUs was measured from December 16, 2021 to December 31, 2022 (the “2021 TSR RSU Performance Period”). Following the 2021 TSR RSU Performance Period, the Organization and Compensation Committee engaged Aon to determine the Company’s performance mercantile. Based on Aon’s determination of the Company’s percentile performance within the Nasdaq Biotechnology Index, the Organization and Compensation Committee determined that none of the 2021 TSR RSUs that were granted to our senior executives and NEOs would vest. Accordingly, all 2021 TSR RSUs granted to our executives were cancelled.
The performance criteria associated with each NEOof the performance stock options and RSUs granted to our senior executives and NEOs in 2022 have not been met and thus, vesting of these performance-based stock options and RSUs has not occurred. If the performance criteria associated with these performance stock options and RSUs are not satisfied within three years of grant, the equity awards will be cancelled.
OTHER COMPENSATION POLICIES AND PRACTICES
Severance and Change of Control Benefits
If the employment of an NEO is terminated by us without cause or by the executive for a designated good reason outside of the context of a change of control transaction, the executive would be entitled to severance benefits under the applicable agreement he or she entered into with the Company. Generally, these severance benefits include a cash severance payment based on the executive’s then-current annual base salary and the amount of his or her target annual incentive bonus, payment of COBRA premiums for up to a maximum of eighteen (18) months, and an additional twelve-month period to exercise vested stock options (an eighteen-month
period for Mr. Robin, and a three-month period for Mr. Wilson and Dr. Zalevsky). In order to attract and retain these NEOs in a competitive environment for highly skilled senior executive talent in the biotechnology and pharmaceutical industry and to provide an incentive to obtain a broad release of claims in favor of the Company, we determined it was often necessary to offer severance benefits in the case of a termination without cause or constructive termination outside the context of a change of control transaction. Many companies provide severance benefits for similar types of terminations of employment, and we believe that it is important for us to offer these severance benefits in order to continue to provide a competitive total compensation program. These NEOs would also be entitled to certain termination benefits upon a termination of employment because of death or disability.
53
We also maintain a Change of Control Severance Benefit Plan (the “CIC Plan”) that provides the NEOs with certain severance benefits if their employment is terminated in connection with a change of control. The CIC Plan was originally established in 2006, and no amendments have been made to the plan since that time that would increase the severance benefits available under the CIC Plan. Severance benefits under the CIC Plan are structured on a “double-trigger” basis, meaning that the executive must experience a termination without cause or resign for a specifically defined good reason in connection with the change of control in order for severance benefits to become payable under the CIC Plan. Like the severance benefits under the letter agreements, we believe that these change of control severance benefits are an important element of a competitive total compensation program. Additionally, we believe that providing change of control benefits should eliminate, or at least reduce, any reluctance of our NEOs and other key employees covered by the CIC Plan to diligently consider and pursue potential change of control opportunities that may be in the best interests of our stockholders. At the same time, by providing change of control benefits only upon the occurrence of an additional triggering event occurring in connection with the change of control transaction resulting in a job loss, we believe that this CIC Plan helps preserve the value of our key personnel for any potential acquiring company.
Under the CIC Plan, the executive would be entitled to accelerated equity award vesting upon a termination described above. The other severance benefits under the CIC Plan are generally similar to the severance benefits described above; however, Mr. Robin’s cash severance would cover the two-year period following termination and Company-paid COBRA coverage would be eighteen months. Outplacement services received within twelve months following separation, up to a maximum of $5,000, are provided to all participants. In addition, each of the NEOs would be entitled to full equity vesting and, except for Dr. Zalevsky, a “gross up” payment for any excise taxes imposed under Section 4999 of the Internal Revenue Code once a 10% cutback threshold is exceeded. The excise tax gross-up was included in the CIC Plan as originally adopted in 2006 to make the participants whole for any adverse tax consequences to which they may become subject under Section 4999 of the Internal Revenue Code and to avoid unintended differences in net severance based on individual factors like the date of hire and past option exercise decisions, which preserves the level of change of control severance protections that we have determined to be appropriate. At the time the CIC Plan was established, we believed this excise tax gross-up protection was a reasonable part of a competitive total compensation package and generally consistent with industry practice at the time. On April 5, 2011, the board of directors amended the CIC Plan to eliminate any “gross up” payments for any excise taxes imposed under Section 4999 of the Internal Revenue Code for participants who became eligible to participate in the CIC Plan on or after January 1, 2010. The board of directors decided to eliminate this tax gross-up provision under the plan for new participants based on its review of current industry practices.
The “Potential Payments Upon Termination or Change of Control” section below describes and quantifies the severance and other benefits potentially payable to the NEOs.
OTHER BENEFITS
We believe that establishing competitive benefit packages for employees is an important factor in attracting and retaining highly-qualified personnel, including the NEOs. The NEOs are eligible to participate in all of our employee benefit plans, such as medical, dental, vision, group life, disability insurance, commuting and parking benefits, wellness benefits, travel and business meeting expenses paid or reimbursed, employee stock purchase plan and the 401(k) plan, in each case generally on the same basis as other employees. We do not offer a tax-qualified defined-benefit pension plan or any non-qualified defined benefit retirement plans, nor do we provide material perquisites to our executives. In 2022,2023, we offered Mr. Robin third party local ground transportation and tax gross ups for such expenses. These ground transportation benefits serve business purposes, such as allowing Mr. Robin to safely increase his productivity by attending to business matters while in transit. We also offered our NEO’s parking passes for use when
commuting to the Company’s office. The parking benefits serve business purposes by facilitating our NEO’s commutes and increases their productivity. See the Summary Compensation Table below for additional information.
OTHER COMPENSATION POLICIES AND PRACTICES
CLAWBACK POLICY
54
are required to revise ourprepare a material financial results,restatement, we maywill recover from any executive officer any incentive compensation erroneously paid or awarded in excess of what would have been based on the revised financial results.restated financials. This policy applies to any incentive compensation that is either granted or paid at any time during the period of three full fiscal years prior to the date on which the financial results applicable to such award or payment are revised.restated.
POLICY PROHIBITING HEDGING
Our Security Trading Policy prohibits our employees, our executive officers, and the non-employee members of our board of directors from short-term trading, options trading, trading on margin, share pledging, and all hedging transactions with respect to our securities.
STOCK OWNERSHIP GUIDELINES
Effective January 1, 2019 the Organization and Compensation Committee approved ownership guidelines for our executive officers, such that the CEO should own shares of our common stock equal to at least three times his or her base salary, and the NEOs should own shares of our common stock equal to at least one time their base salary. The minimum stock ownership level is to be achieved by each executive officer within five years of the date of his or her appointment to executive officer. As of December 31, 2022,2023, each NEO met the minimum stock ownership guidelines or was within the five-year grace period provided by the plan.
TAX AND ACCOUNTING CONSIDERATIONS
Deductibility of Executive Compensation
Generally, Section 162(m) of the Code (“Section 162(m)”) disallows a federal income tax deduction for public corporations of remuneration in excess of $1 million paid in any fiscal year to certain specified executive officers. For taxable years beginning before January 1, 2018 (i) these executive officers consisted of a public corporation’s chief executive officer and up to three other executive officers (other than the chief financial officer) whose compensation is required to be disclosed to stockholders under the Exchange Act because they are our most highly-compensated executive officers and (ii) qualifying “performance-based compensation” was not subject to this deduction limit if specified requirements are met.
Pursuant to the Tax Cuts and Jobs Act of 2017, which was signed into law on December 22, 2017 (the “Tax Act”), for taxable years beginning after December 31, 2017, the remuneration of a public corporation’s chief financial officer is also subject to the deduction limit. In addition, subject to certain transition rules (which apply to remuneration provided pursuant to written binding contracts which were in effect on November 2, 2017 and which are not subsequently modified in any material respect), for taxable years beginning after December 31, 2017, the exemption from the deduction limit for “performance-based compensation” is no longer available. In addition, under the Tax Act, once an executive becomes a “covered employee” under Section 162(m), the individual will continue to be a “covered employee” as long as he or she remains employed by the company. Consequently, for fiscal years beginning after December 31, 2017, all remuneration in excess of $1 million paid to a covered executive will not be deductible unless it qualifies for transitional relief applicable to certain binding, written performance-based compensation arrangements that were in place as November 2, 2017 or transitional relief for applicable to certain newly public companies. These changes will cause more of our compensation to be non-deductible under Section 162(m) in the future and will eliminate the Company’s ability to structure performance-based awards to be exempt from Section 162(m).
Furthermore, on March 11, 2021, The American Rescue Plan Act of 2021 (the “ARPA”) was signed into law to assist in the economic and health recovery brought on by the COVID-19 pandemic. Beginning on or after January 1, 2027, the ARPA expands the applicability of Section 162(m) to also include the next five highest paid corporate officers so that the total number of covered employees subject to the $1 million deduction limitation will at least be 10.
In designing our executive compensation program and determining the compensation of our executive officers, including our NEOs, the Organization and Compensation Committee considers a variety of factors, including the potential impact of the Section 162(m) deduction limit. While the Organization and Compensation Committee is mindful of the benefit of the full deductibility of compensation, it believes that we should not be constrained by the requirements of Section 162(m) where those requirements would impair our flexibility in
55
compensating our executive officers in a manner that can best promote our corporate objectives. Therefore, the Organization and Compensation Committee has not adopted a policy that would require that all compensation be deductible, though it does consider the deductibility of compensation when making compensation decisions. The Organization and Compensation Committee may authorize compensation payments that are not fully tax deductible if it believes that such payments are appropriate to attract and retain executive talent or meet other business objectives.
56
Accounting for Stock-Based Compensation
We follow FASB ASC Topic 718 for our stock-based compensation awards. FASB ASC Topic 718 requires us to measure the compensation expense for all share-based payment awards made to our employees and non-employee members of our board of directors, including options to purchase shares of our common stock and other stock awards, based on the grant date “fair value” of these awards. This cost is recognized as an expense following the straight-line attribution method over the requisite service period. This calculation is performed for accounting purposes and reported in the executive compensation tables required by the federal securities laws, even though the recipient of the awards may never realize any value from such awards.
The material in this report is being furnished and shall not be deemed “filed” with the SEC for purposes of Section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall the material in this section be deemed to be “soliciting material” or incorporated by reference in any registration statement or other document filed with the SEC under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as otherwise expressly stated in such filing.
The Organization and Compensation Committee has reviewed the Compensation Discussion and Analysis and discussed it with management. Based on its review and discussions with management, the committee recommended to our board of directors that the Compensation Discussion and Analysis be included in our annual report on Form 10-K for the fiscal year ended December 31, 20222023 and in our 20232024 proxy statement. This report is provided by the following independent directors, who currently comprise the committee:
Myriam J. Curet, M.D.
Name and Principal Position (a) | | Year (b) | | Salary ($) (c) | | Bonus ($) (d) | | Stock Awards ($)(4)(6)(7)(8)(9) (e) | | Option Awards ($)(5)(6)(8)(9) (f) | | Non-Equity Incentive Plan Compensation ($)(10) (g) | | All Other Compensation ($) (i) | | Total Compensation ($) (j) | | Year (b) | | Salary ($) (c) | | Bonus ($) (d) | | Stock Awards ($)(3)(6)(7)(8) (e) | | Option Awards ($)(4)(5)(6)(7) (f) | | Non-Equity Incentive Plan Compensation ($)(9) (g) | | All Other Compensation ($) (i) | | Total Compensation ($) (j) | ||||||||||||||||
Howard W. Robin President and Chief Executive Officer | | | 2022 | | 1,084,590 | | | 4,032,225 | | 5,409,410 | | 650,754 | | 98,821(11) | | 11,275,830 | | | 2023 | | 1,084,590 | | — | | — | | 927,160 | | 1,030,361 | | 93,465(10) | | 3,135,576 | |||||||||||||||
| | 2021 | | 1,053,000 | | | 5,691,794 | | 3,326,492 | | 1,000,350 | | 81,980 | | 11,153,616 | | 2022 | | 1,084,590 | | — | | 4,032,255 | | 5,409,410 | | 650,754 | | 98,821 | | 11,275,830 | ||||||||||||||||||
| | 2020 | | 1,053,000 | | | 4,996,266 | | 4,004,108 | | 1,092,000 | | 95,775 | | 11,241,149 | | 2021 | | 1,053,000 | | — | | 5,691,794 | | 3,326,492 | | 1,000,350 | | 81,980 | | 11,153,616 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | |||||||||||||||||||||||||||||||||
Jillian B. Thomsen(1) Chief Financial Officer and Chief Accounting Officer | | 2022 | | 522,200 | | | 1,629,478 | | 2,185,994 | | 198,000 | | 22,645(12) | | 4,558,317 | |||||||||||||||||||||||||||||||||
Sandra Gardiner(1) Interim Chief Financial Officer | | 2023 | | — | | — | | — | | — | | — | | 376,300(11) | | 376,300 | ||||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | |||||||||||||||||||||||||||||||||
Mark A. Wilson Chief Legal Officer | | | 2022 | | 527,500 | | | 1,629,478 | | 2,185,994 | | 194,400 | | 28,093(13) | | 4,565,465 | | | 2023 | | 540,000 | | — | | — | | 231,790 | | 307,800 | | 20,878(12) | | 1,100,468 | |||||||||||||||
| | 2021 | | 500,000 | | | 1,707,078 | | 998,320 | | 287,500 | | 10,763 | | 3,503,661 | | 2022 | | 527,500 | | — | | 1,629,478 | | 2,185,994 | | 194,400 | | 28,093 | | 4,565,465 | ||||||||||||||||||
| | 2020 | | 500,000 | | | 1,604,292 | | 1,071,122 | | 270,000 | | 10,492 | | 3,455,906 | | 2021 | | 500,000 | | — | | 1,707,078 | | 998,320 | | 287,500 | | 10,763 | | 3,503,661 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | |||||||||||||||||||||||||||||||||
Jonathan Zalevsky, Ph.D. Chief Research and Development Officer | | | 2022 | | 703,490 | | | 1,325,673 | | 1,778,436 | | 253,256 | | 289,625(14) | | 4,350,480 | | | 2023 | | 703,490 | | | — | | 178,300 | | 400,989 | | 490,585(13) | | 1,773,364 | ||||||||||||||||
| | 2021 | | 683,000 | | | 2,391,094 | | 1,397,498 | | 471,270 | | 8,385 | | 4,951,247 | | 2022 | | 703,490 | | — | | 1,325,673 | | 1,778,436 | | 253,256 | | 289,625 | | 4,350,480 | ||||||||||||||||||
| | 2020 | | 683,000 | | | 2,247,676 | | 1,501,672 | | 500,000 | | 9,149 | | 4,941,497 | | 2021 | | 683,000 | | — | | 2,391,094 | | 1,397,498 | | 471,270 | | 8,385 | | 4,951,247 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | |||||||||||||||||||||||||||||||||
Gil M. Labrucherie(2) Former Chief Operating Officer and Chief Financial Officer | | | 2022 | | 576,733 | | | — | | — | | — | | 10,972(15) | | 587,705 | ||||||||||||||||||||||||||||||||
| | 2021 | | 788,000 | | | 2,391,094 | | 1,397,498 | | 679,650 | | 23,314 | | 5,279,556 | ||||||||||||||||||||||||||||||||||
| | 2020 | | 788,000 | | | 2,247,676 | | 1,501,672 | | 721,000 | | 27,574 | | 5,285,922 | ||||||||||||||||||||||||||||||||||
| | | | | | | | | |||||||||||||||||||||||||||||||||||||||||
John Northcott(3) Former Chief Commercial Officer | | | 2022 | | 441,662 | | | — | | — | | — | | 923,019(16) | | 1,364,681 | ||||||||||||||||||||||||||||||||
| | 2021 | | 675,000 | | | 1,707,078 | | 998,320 | | 388,125 | | 7,950 | | 3,776,473 | ||||||||||||||||||||||||||||||||||
| | 2020 | | 675,000 | | | 1,604,292 | | 1,071,122 | | 364,500 | | 117,884 | | 3,832,798 | ||||||||||||||||||||||||||||||||||
Jillian B. Thomsen(2) Former Chief Financial Officer and Chief Accounting Officer | | | 2023 | | 355,760 | | — | | — | | — | | — | | 883,999(14) | | 1,239,759 | |||||||||||||||||||||||||||||||
| | 2022 | | 522,200 | | — | | 1,629,478 | | 2,185,994 | | 198,000 | | 22,645 | | 4,558,317 | |||||||||||||||||||||||||||||||||
| (1) | Ms. |
| (2) | Ms. Thomsen served as our Chief Financial Officer and Chief Accounting Officer |
| (3) |
| Amounts reported represent the aggregate grant date fair value of RSU awards computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, Compensation-Stock Compensation (“FASB ASC Topic 718”), based on the closing price of the Company’s common stock on the grant date and excluding the effects of estimated forfeitures. For a complete description of the assumptions made in determining the valuation, please refer to (i) Note |
| Amounts reported represent the aggregate grant date fair value of the stock options granted in the applicable year computed in accordance with FASB ASC Topic 718, which excludes the effects of estimated forfeitures. For a complete description of the assumptions made in determining the valuation, please refer to (i) Note 10 (Stock-Based Compensation) to our audited financial statements in our annual report on Form 10-K for the fiscal year ended December 31, 2023 and (ii) similar footnotes to our audited financial statements in our annual reports on Form 10-K for prior years when the awards were granted. |
| (6) | 50% of the annual equity |
59
| (7) | 63% of the annual equity awards granted to the NEOs in 2021 were performance-based with, 37% of the annual equity award granted to the NEOs in 2021 vesting only to the extent a specified performance-based vesting condition is satisfied within 5 years of grant and 19% of the annual equity award vesting at 2 and 3 years if certain total shareholder return criteria are satisfied on December 31, 2023. If the performance-based vesting condition is satisfied, then the performance-based equity awards also remain subject to a time-based vesting requirement. The amounts reported in the “Stock Awards” and “Option Awards” column of the table for 2021 include the grant date fair value of performance-based RSUs and stock options, as applicable for the year, based on the probable outcome (determined as of the grant date in accordance with generally accepted accounting principles) of the performance-based conditions applicable to the awards which assumes maximum achievement of the underlying performance conditions. |
| (8) | A portion of the 2021 performance-based equity awards vest only to the extent a specified performance-based vesting condition is satisfied by June 1, 2022. Since the performance-based vesting condition was not met, all RSUs granted under such performance grants were forfeited and cancelled in 2022. However, the amounts reported in the “Stock Awards” column of the table for 2021 include the grant date fair value of such RSUs based on the probable outcome (determined as of the grant date in accordance with generally accepted accounting principles) of the performance-based conditions applicable to the awards which assumes maximum achievement of the underlying performance conditions. |
| (9) |
| Amounts reported for |
| Includes (i) life insurance premiums of |
| (11) | Consists of consulting fees paid to FLG Partners, LLC, pursuant to the Gardiner Consulting Agreement, in connection with Ms. Gardiner’s services as Interim Chief Financial Officer. |
| (12) | Includes (i) life insurance premiums of |
| (13) | Includes (i) life insurance premiums of |
| Includes (i) life insurance premiums of |
Each of the NEOs, other than Ms. Gardiner, has entered into our standard form of employment agreement and an offer letter or letter agreement. The form of employment agreement provides for protective covenants with respect to confidential information, intellectual property and assignment of inventions and also sets forth other standard terms and conditions of employment. The offer letter agreements do not provide for any minimum or guaranteed term of employment. The letter agreementsTo the extent an NEO entered into by each ofa letter agreement with us, the NEOsletter agreement(s) establish the compensation arrangements following separation from us under certain circumstances. Please see “Potential Payments upon Termination or Change of Control” below for more information on these separation arrangements.
The following table shows, for the fiscal year ended December 31, 2022,2023, certain information regarding grants of plan-based awards to the NEOs.NEOs(1).
Name (a) | | | Grant Date (b) | | | Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) | | | Estimated Future Payouts Under Equity Incentive Plan Awards(2) | | | All Other Stock Awards: Number of Shares of Stock or Units (#)(3) (i) | | | All Other Option Awards: Number of Securities Underlying Options (#)(4) (j) | | | Exercise or Base Price of Option Awards ($/sh)(5) (k) | | | Grant Date Fair Value of Stock and Option Awards ($)(6) (l) | ||||||||||||
| | Threshold ($) (c) | | | Target ($) (d) | | | Maximum ($) (e) | | | Threshold (#) (f) | | | Target (#) (g) | | | Maximum (#) (h) | | ||||||||||||||||
Howard W. Robin | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
Annual Incentive Award | | | N/A | | | | | 1,084,590 | | | 2,169,180 | | | | | | | | | | | | | | | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | 410,625 | | | | | | | | | | | 2,016,169 | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | | | | | 410,625 | | | | | | | 2,016,169 | ||||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | 821,250 | | | | | | | | | $4.91 | | | 2,704,705 | |||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | | | | | | | 821,250 | | | $4.91 | | | 2,704,705 | |||||||
| | | | | | | | | | | | | | | | | | | | | | | ||||||||||||
Jillian B. Thomsen | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
Annual Incentive Award | | | N/A | | | | | 330,000 | | | 660,000 | | | | | | | | | | | | | | | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | 165,938 | | | | | | | | | | | 814,756 | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | | | | | 165,938 | | | | | | | 814,756 | ||||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | 331,875 | | | | | | | | | $4.91 | | | 1,092,997 | |||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | | | | | | | 331,875 | | | $4.91 | | | 1,092,997 | |||||||
| | | | | | | | | | | | | | | | | | | | | | | ||||||||||||
Mark A. Wilson | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
Annual Incentive Award | | | N/A | | | | | 324,000 | | | 648,000 | | | | | | | | | | | | | | | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | 165,938 | | | | | | | | | | | 814,756 | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | | | | | 165,938 | | | | | | | 814,756 | ||||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | 331,875 | | | | | | | | | $4.91 | | | 1,092,997 | |||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | | | | | | | 331,875 | | | $4.91 | | | 1,092,997 | |||||||
| | | | | | | | | | | | | | | | | | | | | | | ||||||||||||
Jonathan Zalevsky, Ph.D. | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
Annual Incentive Award | | | N/A | | | | | 422,094 | | | 844,188 | | | | | | | | | | | | | | | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | 135,000 | | | | | | | | | | | 662,850 | ||||||||
Restricted Stock Units | | | 8/15/2022 | | | | | | | | | | | | | | | 135,000 | | | | | | | 662,850 | ||||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | 270,000 | | | | | | | | | $4.91 | | | 889,218 | |||||||
Stock Options | | | 8/15/2022 | | | | | | | | | | | | | | | | | 270,000 | | | $4.91 | | | 889,218 | |||||||
Name (a) | | | Grant Date (b) | | | Estimated Future Payouts Under Non-Equity Incentive Plan Awards(2) | | | Estimated Future Payouts Under Equity Incentive Plan Awards(3) | | | All Other Stock Awards: Number of Shares of Stock or Units (#) (i) | | | All Other Option Awards: Number of Securities Underlying Options (#)(4) (j) | | | Exercise or Base Price of Option Awards ($/sh)(5) (k) | | | Grant Date Fair Value of Stock and Option Awards ($)(6) (l) | ||||||||||||
| | Threshold ($) (c) | | | Target ($) (d) | | | Maximum ($) (e) | | | Threshold (#) (f) | | | Target (#) (g) | | | Maximum (#) (h) | | ||||||||||||||||
| Howard W. Robin | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
| Annual Incentive Award | | | N/A | | | | | 1,084,590 | | | 2,169,180 | | | | | | | | | | | | | | | ||||||||
| Stock Options | | | 12/13/2023 | | | | | | | | | | | 1,300,000 | | | | | | | | | $0.50 | | | 463,580 | |||||||
| Stock Options | | | 12/13/2023 | | | | | | | | | | | | | | | | | 1,300,000 | | | $0.50 | | | 463,580 | |||||||
| | | | | | | | | | | | | | | | | | | | | | | ||||||||||||
| Mark A. Wilson | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
| Annual Incentive Award | | | N/A | | | | | 324,000 | | | 648,000 | | | | | | | | | | | | | | | ||||||||
| Stock Options | | | 12/13/2023 | | | | | | | | | | | 325,000 | | | | | | | | | $0.50 | | | 115,895 | |||||||
| Stock Options | | | 12/13/2023 | | | | | | | | | | | | | | | | | 325,000 | | | $0.50 | | | 115,895 | |||||||
| | | | | | | | | | | | | | | | | | | | | | | ||||||||||||
Jonathan Zalevsky | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
| Annual Incentive Award | | | N/A | | | | | 422,094 | | | 844,188 | | | | | | | | | | | | | | | ||||||||
| Stock Options | | | 12/13/2023 | | | | | | | | | | | 250,000 | | | | | | | | | $0.50 | | | 89,150 | |||||||
| Stock Options | | | 12/13/2023 | | | | | | | | | | | | | | | | | 250,000 | | | $0.50 | | | 89,150 | |||||||
| (1) | Ms. Gardiner did not receive any equity grants in 2023 and does not participate in our Incentive Compensation Plan. Ms. Thomsen, our former Chief Financial Officer and Chief Accounting Officer, did not receive any equity grants in 2023 because her employment with the Company ended on June 16, 2023. |
| (2) | Amounts reported represent the potential short-term incentive compensation amounts payable for our |
| (4) | These stock option grants are subject to a three-year monthly pro-rata time vesting requirement. |
| (5) |
| (6) | Refer to Note |
Description of Plan-Based Awards
Time-Based Stock Options. Each time-based stock option granted to the NEOs during 20222023 may be exercised to purchase the designated number of shares of our common stock at an exercise price equal to the closing price of the underlying common stock on the grant date. Each NEO’s time-based stock option award granted in 20222023 has a maximum term of eight (8) years and is subject to a vesting schedule that requires the executive’s continued service through the vesting date. The 20222023 stock option awards granted to the NEOs will vest on a monthly pro-rata basis over a three-year period following the grant date, subject to the executive’s continued service through the vesting date.
Any stock options or RSUs that are unvested upon an NEO’s termination of continuous employment or services will be forfeited without any value, unless the termination of continuous service is a result of death, in which event, subject to any restrictions in the stock option or RSU agreement or equity incentive plan, the stock option would become fully vested and exercisable as of the date of death and the RSU would become fully vested and released as of the date of death. For Mr. Robin, in accordance with his letter agreements, if any stock options are unvested upon a termination of continuous employment as a result of a disability, 50% of the unvested stock options would become fully vested and exercisable as of the date of termination. In accordance with the letter agreements for the NEOs described above, any stock options that are vested upon termination of continuous service by us without cause or by the executive for a good reason resignation (as defined in the CIC Plan) will remain outstanding and exercisable for eighteen (18) months for Mr. Robin and three (3) months for Ms. Thomsen, Mr. Wilson and Dr. Zalevsky. This exercise period is also twelve (12) months if the termination of employment or continuous services is because of disability and is eighteen (18) months if the termination is a result of death. We also have the discretion to extend the applicable exercise period in connection with other terminations of employment. Any vested stock options that are not exercised within the applicable post-termination of employment exercise period will terminate.
Under the terms of the 2017 Plan, if there is a change of control of the Company, outstanding awards granted under the plan will generally become fully vested and, in the case of stock options, exercisable, unless the Organization and Compensation Committee provides for the substitution, assumption, exchange or other continuation of the outstanding awards. Any stock options that become vested in connection with a change of control generally must be exercised prior to the change of control, or they will be cancelled in exchange for the right to receive a cash payment in connection with the change of control transaction. In addition, outstanding awards held by our NEOs may vest, upon certain terminations of the NEO’s employment without cause or for a good reason resignation in connection with a change of control and in connection with terminations of employment resulting from disability or death. Please see the “Potential Payments Upon Termination or Change of Control” section below for a description of the vesting that may occur in such circumstances.
In 20222023 each NEO’s stock option and RSU award was granted under, and is subject to the terms of, the 2017 Plan. The plan is administered by the Organization and Compensation Committee, and this committee has the ability to interpret and make all required determinations under the plan. This authority includes making required proportionate adjustments to outstanding equity awards to reflect certain corporate transactions and making provision to ensure that participants satisfy any required withholding taxes.
The NEOs are not entitled to any dividend equivalent rights on their stock option or RSU awards, and stock option and RSU awards are generally only transferable to a beneficiary of an NEO upon his death.
62
Short-Term Incentive Compensation. AllFor 2024, all of the NEOs werewill be eligible to earn a short-term incentive compensation payment under the Incentive Compensation Plan or, for Mr. Robin, under an arrangement that mirrors the Incentive Compensation Plan in his amended and restated offer letter effective as of December 1, 2008. These opportunities are reflected in the “Estimated Possible Payouts Under Non-Equity Incentive Plan Awards” columns of the table above. Please see “Compensation Discussion and Analysis—Current Executive Compensation Program Elements—Short-Term Incentive Compensation” for a description of the material terms of the Incentive Compensation Plan and Mr. Robin’s related short-term incentive compensation arrangement. In 2023 each NEO received an incentive cash compensation payment for the 2023 performance period based on the Company’s achievement of corporate performance objectives.
63
The following table includes certain information with respect to the value of all unexercised stock options and outstanding equity awards previously awarded to the NEOs as of December 31, 2022.2023.
| | | | Option Awards | | Stock Awards | | | | Option Awards | | Stock Awards | |||||||||||||||||||||||||||||||||||||||||||||||||
Name (a) | | Grant Date | | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#)(1) (d) | | Option Exercise Price ($) (e) | | Option Expiration Date(2) (f) | | Number of Shares or Units of Stock That Have Not Vested (#) (g) | | Market Value of Shares or Units of Stock That Have Not Vested ($)(3) (h) | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (i) | | Equity Incentive Plan Awards: Market or Payout value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(3) (j) | | Grant Date | | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) (d) | | Option Exercise Price ($) (e) | | Option Expiration Date(3) (f) | | Number of Shares or Units of Stock That Have Not Vested (#) (g) | | Market Value of Shares or Units of Stock That Have Not Vested ($)(4) (h) | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (i) | | Equity Incentive Plan Awards: Market or Payout value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(4) (j) | ||||||||||||||||||||
Howard W. Robin | | 12/15/2015 | | 56,250 | | | | 15.55 | | 12/14/2023 | | | | | | 12/13/2016 | | 137,500 | | | | 12.24 | | 12/12/2024 | | | | | ||||||||||||||||||||||||||||||||
| | 12/15/2015 | | 56,250 | | | | 15.55 | | 12/14/2023 | | | | | | 12/13/2016 | | 137,500 | | | | 12.24 | | 12/12/2024 | | | | | |||||||||||||||||||||||||||||||||
| | 12/13/2016 | | 137,500 | | | | 12.24 | | 12/12/2024 | | | | | | 12/15/2017 | | 151,250 | | | | 56.90 | | 12/14/2025 | | | | | |||||||||||||||||||||||||||||||||
| | 12/13/2016 | | 137,500 | | | | 12.24 | | 12/12/2024 | | | | | | 12/15/2017 | | 151,250 | | | | 56.90 | | 12/14/2025 | | | | | |||||||||||||||||||||||||||||||||
| | 12/15/2017 | | 151,250 | | | | 56.90 | | 12/14/2025 | | | | | | 12/14/2018 | | 138,350 | | | | 36.51 | | 12/13/2026 | | | | | |||||||||||||||||||||||||||||||||
| | 12/15/2017 | | 151,250 | | | | 56.90 | | 12/14/2025 | | | | | | 12/14/2018 | | 138,350 | | | | 36.51 | | 12/13/2026 | | | | | |||||||||||||||||||||||||||||||||
| | 12/14/2018 | | 138,350 | | | | 36.51 | | 12/13/2026 | | | | | | 12/12/2019 | | 167,650 | | | | 21.79 | | 12/11/2027 | | | | | |||||||||||||||||||||||||||||||||
| | 12/14/2018 | | 138,350 | | | | 36.51 | | 12/13/2026 | | | | | | 12/12/2019 | | 167,650 | | | | 21.79 | | 12/11/2027 | | | | | |||||||||||||||||||||||||||||||||
| | 12/12/2019 | | 125,742 | | 41,908(4) | | | 21.79 | | 12/11/2027 | | | | | | 12/12/2019 | | 44,600 | | | | 21.79 | | 12/11/2027 | | | | | ||||||||||||||||||||||||||||||||
| | 12/12/2019 | | 125,737 | | 41,913(5) | | | 21.79 | | 12/11/2027 | | | | | | 12/18/2020 | | 142,987 | | 47,663(5) | | | 18.75 | | 12/17/2028 | | | | | |||||||||||||||||||||||||||||||
| | 12/12/2019 | | 33,450 | | 11,150(5) | | | 21.79 | | 12/11/2027 | | | | | | 12/18/2020 | | | | 190,650(6) | | 18.75 | | 12/17/2028 | | | | | ||||||||||||||||||||||||||||||||
| | 12/18/2020 | | 95,325 | | 95,325(5) | | | 18.75 | | 12/17/2028 | | | | | | 12/18/2020 | | | | | | | | | 106,650(8) | | 60,791 | |||||||||||||||||||||||||||||||||
| | 12/18/2020 | | | | 190,650(6) | | 18.75 | | 12/17/2028 | | | | | | 12/16/2021 | | 111,875 | | 111,875(5) | | | 13.22 | | 12/15/2029 | | | | | ||||||||||||||||||||||||||||||||
| | 12/18/2020 | | | | | | | 35,550(7) | | 80,343 | | | | 12/16/2021 | | | | 223,750(6) | | 13.22 | | 12/15/2029 | | | | | ||||||||||||||||||||||||||||||||||
| | 12/18/2020 | | | | | | | | | 106,650(8) | | 241,029 | | 12/16/2021 | | | | | | | 42,017(7) | | 23,950 | | | |||||||||||||||||||||||||||||||||||
| | 12/16/2021 | | 55,937 | | 167,813(5) | | | 13.22 | | 12/15/2029 | | | | | | 12/16/2021 | | | | | | | | | 126,050(8) | | 71,849 | |||||||||||||||||||||||||||||||||
| | 12/16/2021 | | | | 223,750(6) | | 13.22 | | 12/15/2029 | | | | | | 08/15/2022 | | 365,000 | | 456,250(9) | | | 4.91 | | 08/14/2030 | | | | | ||||||||||||||||||||||||||||||||
| | 12/16/2021 | | | | | | | 84,034(7) | | 189,917 | | | | 08/15/2022 | | | | 821,250(10) | | 4.91 | | 08/14/2030 | | | | | ||||||||||||||||||||||||||||||||||
| | 12/16/2021 | | | | | | | | | 126,050(8) | | 284,873 | | 08/15/2022 | | | | | | | 239,532(7) | | 136,533 | | | |||||||||||||||||||||||||||||||||||
| | 12/16/2021 | | | | | | | | | 84,300(9) | | 190,518 | | 08/15/2022 | | | | | | | | | 410,625(8) | | 234,056 | |||||||||||||||||||||||||||||||||||
| | 08/15/2022 | | 91,250 | | 730,000(10) | | | 4.91 | | 08/14/2030 | | | | | | 12/13/2023 | | | 1,300,000(9) | | | 0.50 | | 12/12/2031 | | | | | ||||||||||||||||||||||||||||||||
| | 08/15/2022 | | | | 821,250(11) | | 4.91 | | 08/14/2030 | | | | | | 12/13/2023 | | | | 1,300,000(10) | | 0.50 | | 12/12/2031 | | | | | |||||||||||||||||||||||||||||||||
| | 08/15/2022 | | | | | | | 376,407(7) | | 850,680 | | | ||||||||||||||||||||||||||||||||||||||||||||||||
| | 08/15/2022 | | | | | | | | | 410,625(8) | | 928,013 | ||||||||||||||||||||||||||||||||||||||||||||||||
Jillian B. Thomsen | | 12/15/2015 | | 18,750 | | | | 15.55 | | 12/14/2023 | | | | | ||||||||||||||||||||||||||||||||||||||||||||||
Sandra Gardiner(1) | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | ||||||||||||||||||||||||||||||||||||||||
| Mark A. Wilson | | 7/15/2016 | | 10,000 | | | | 15.45 | | 7/14/2024 | | | | | ||||||||||||||||||||||||||||||||||||||||||||||
| | 12/15/2015 | | 18,750 | | | | 15.55 | | 12/14/2023 | | | | | | 12/13/2016 | | 20,000 | | | | 12.24 | | 12/12/2024 | | | | | |||||||||||||||||||||||||||||||||
| | 12/13/2016 | | 37,500 | | | | 12.24 | | 12/12/2024 | | | | | | 12/15/2017 | | 110,000 | | | | 56.90 | | 12/14/2025 | | | | | |||||||||||||||||||||||||||||||||
| | 12/13/2016 | | 37,500 | | | | 12.24 | | 12/12/2024 | | | | | | 12/15/2017 | | 42,000 | | | | 56.90 | | 12/14/2025 | | | | | |||||||||||||||||||||||||||||||||
| | 12/15/2017 | | 26,250 | | | | 56.90 | | 12/14/2025 | | | | | | 12/14/2018 | | 10,225 | | | | 36.51 | | 12/13/2026 | | | | | |||||||||||||||||||||||||||||||||
| | 12/15/2017 | | 26,250 | | | | 56.90 | | 12/14/2025 | | | | | | 12/12/2019 | | 35,800 | | | | 21.79 | | 12/11/2027 | | | | | |||||||||||||||||||||||||||||||||
| | 12/14/2018 | | 22,150 | | | | 36.51 | | 12/13/2026 | | | | | | 12/12/2019 | | 35,800 | | | | 21.79 | | 12/11/2027 | | | | | |||||||||||||||||||||||||||||||||
| | 12/14/2018 | | 22,150 | | | | 36.51 | | 12/13/2026 | | | | | | 12/12/2019 | | 11,600 | | | | 21.79 | | 12/11/2027 | | | | | |||||||||||||||||||||||||||||||||
| | 12/12/2019 | | 19,952 | | 6,648(4) | | | 21.79 | | 12/11/2027 | | | | | | 12/18/2020 | | 38,250 | | 12,750(5) | | | 18.75 | | 12/17/2028 | | | | | |||||||||||||||||||||||||||||||
| | 12/12/2019 | | 19,950 | | 6,650(5) | | | 21.79 | | 12/11/2027 | | | | | | 12/18/2020 | | | | 51,000(6) | | 18.75 | | 12/17/2028 | | | | | ||||||||||||||||||||||||||||||||
| | 12/12/2019 | | 8,850 | | 2,950(5) | | | 21.79 | | 12/11/2027 | | | | | | 12/18/2020 | | | | | | | | | 28,550(8) | | 16,274 | |||||||||||||||||||||||||||||||||
| | 12/18/2020 | | 14,300 | | 14,300(5) | | | 18.75 | | 12/17/2028 | | | | | | 12/16/2021 | | 33,575 | | 33,575(5) | | | 13.22 | | 12/15/2029 | | | | | |||||||||||||||||||||||||||||||
| | 12/18/2020 | | | | 28,600(6) | | 18.75 | | 12/17/2028 | | | | | | 12/16/2021 | | | | 67,150(6) | | 13.22 | | 12/15/2029 | | | | | |||||||||||||||||||||||||||||||||
| | 12/18/2020 | | | | | | | 5,334(7) | | 12,055 | | | | 12/16/2021 | | | | | | | 12,600(7) | | 7,182 | | | |||||||||||||||||||||||||||||||||||
| | 12/18/2020 | | | | | | | | | 16,000(8) | | 36,160 | | 12/16/2021 | | | | | | | | | 37,800(8) | | 21,546 | |||||||||||||||||||||||||||||||||||
| | 08/15/2022 | | 147,500 | | 184,375(9) | | | 4.91 | | 08/14/2030 | | | | | ||||||||||||||||||||||||||||||||||||||||||||||
| | 08/15/2022 | | | | 331,875(10) | | 4.91 | | 08/14/2030 | | | | | |||||||||||||||||||||||||||||||||||||||||||||||
| | 08/15/2022 | | | | | | | 96,798(7) | | 55,175 | | | ||||||||||||||||||||||||||||||||||||||||||||||||
| | 08/15/2022 | | | | | | | | | 165,938(8) | | 94,585 | ||||||||||||||||||||||||||||||||||||||||||||||||
| | 12/13/2023 | | | 325,000(9) | | | 0.50 | | 12/12/2031 | | | | | |||||||||||||||||||||||||||||||||||||||||||||||
| | 12/13/2023 | | | | 325,000(10) | | 0.50 | | 12/12/2031 | | | | | |||||||||||||||||||||||||||||||||||||||||||||||
64
| | | | | Option Awards | | | Stock Awards | |||||||||||||||||||||||
Name (a) | | | Grant Date | | | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | | | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#)(1) (d) | | | Option Exercise Price ($) (e) | | | Option Expiration Date(2) (f) | | | Number of Shares or Units of Stock That Have Not Vested (#) (g) | | | Market Value of Shares or Units of Stock That Have Not Vested ($)(3) (h) | | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (i) | | | Equity Incentive Plan Awards: Market or Payout value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(3) (j) |
| | | 12/16/2021 | | | 9,400 | | | 28,200(5) | | | | | 13.22 | | | 12/15/2029 | | | | | | | | | ||||||
| | | 12/16/2021 | | | | | | | 37,600(6) | | | 13.22 | | | 12/15/2029 | | | | | | | | | |||||||
| | | 12/16/2021 | | | | | | | | | | | | | 14,134(7) | | | 31,943 | | | | | ||||||||
| | | 12/16/2021 | | | | | | | | | | | | | | | | | 21,200(8) | | | 47,912 | ||||||||
| | | 12/16/2021 | | | | | | | | | | | | | | | | | 14,200(9) | | | 32,092 | ||||||||
| | | 08/15/2022 | | | 36,875 | | | 295,000(10) | | | | | 4.91 | | | 08/14/2030 | | | | | | | | | ||||||
| | | 08/15/2022 | | | | | | | 331,875(11) | | | 4.91 | | | 08/14/2030 | | | | | | | | | |||||||
| | | 08/15/2022 | | | | | | | | | | | | | 152,110(7) | | | 343,769 | | | | | ||||||||
| | | 08/15/2022 | | | | | | | | | | | | | | | | | 165,938(8) | | | 375,020 | ||||||||
Mark A. Wilson | | | 12/15/2015 | | | 15,000 | | | | | | | 15.55 | | | 12/14/2023 | | | | | | | | | ||||||
| | | 7/15/2016 | | | 10,000 | | | | | | | 15.45 | | | 7/14/2024 | | | | | | | | | |||||||
| | | 12/13/2016 | | | 20,000 | | | | | | | 12.24 | | | 12/12/2024 | | | | | | | | | |||||||
| | | 12/15/2017 | | | 110,000 | | | | | | | 56.90 | | | 12/14/2025 | | | | | | | | | |||||||
| | | 12/15/2017 | | | 42,000 | | | | | | | 56.90 | | | 12/14/2025 | | | | | | | | | |||||||
| | | 12/14/2018 | | | 10,225 | | | | | | | 36.51 | | | 12/13/2026 | | | | | | | | | |||||||
| | | 12/12/2019 | | | 26,854 | | | 8,946(4) | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | ||||||
| | | 12/12/2019 | | | 26,850 | | | 8,950(5) | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | ||||||
| | | 12/12/2019 | | | 8,700 | | | 2,900(5) | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | ||||||
| | | 12/18/2020 | | | 25,500 | | | 25,500(5) | | | | | 18.75 | | | 12/17/2028 | | | | | | | | | ||||||
| | | 12/18/2020 | | | | | | | 51,000(6) | | | 18.75 | | | 12/17/2028 | | | | | | | | | |||||||
| | | 12/18/2020 | | | | | | | | | | | | | 9,517(7) | | | 21,509 | | | | | ||||||||
| | | 12/18/2020 | | | | | | | | | | | | | | | | | 28,550(8) | | | 64,523 | ||||||||
| | | 12/16/2021 | | | 16,787 | | | 50,363(5) | | | | | 13.22 | | | 12/15/2029 | | | | | | | | | ||||||
| | | 12/16/2021 | | | | | | | 67,150(6) | | | 13.22 | | | 12/15/2029 | | | | | | | | | |||||||
| | | 12/16/2021 | | | | | | | | | | | | | | | | | 25,300(9) | | | 57,178 | ||||||||
| | | 12/16/2021 | | | | | | | | | | | | | 25,200 (7) | | | 56,952 | | | | | ||||||||
| | | 12/16/2021 | | | | | | | | | | | | | | | | | 37,800(8) | | | 85,428 | ||||||||
| | | 08/15/2022 | | | 36,875 | | | 295,000(10) | | | | | 4.91 | | | 08/14/2030 | | | | | | | | | ||||||
| | | 08/15/2022 | | | | | | | 331,875(11) | | | 4.91 | | | 08/14/2030 | | | | | | | | | |||||||
| | | 08/15/2022 | | | | | | | | | | | | | 152,110(7) | | | 343,769 | | | | | ||||||||
| | | 08/15/2022 | | | | | | | | | | | | | | | | | 165,938(8) | | | 375,020 | ||||||||
| | | | | | | | | | | | | | | | | | | | | |||||||||||
Johnathan Zalevsky, Ph.D. | | | 7/31/2015 | | | 21,875 | | | | | | | 12.61 | | | 7/30/2023 | | | | | | | | | ||||||
| | | 12/15/2015 | | | 29,688 | | | | | | | 15.55 | | | 12/14/2023 | | | | | | | | | |||||||
| | | 5/31/2016 | | | 37,500 | | | | | | | 15.44 | | | 5/30/2024 | | | | | | | | | |||||||
| | | 11/15/2016 | | | 46,875 | | | | | | | 13.93 | | | 11/14/2024 | | | | | | | | | |||||||
| | | 12/13/2016 | | | 15,500 | | | | | | | 12.24 | | | 12/12/2024 | | | | | | | | | |||||||
| | | 3/16/2017 | | | 21,250 | | | | | | | 15.71 | | | 3/14/2025 | | | | | | | | | |||||||
| | | 4/18/2017 | | | 36,459 | | | | | | | 18.585 | | | 4/17/2025 | | | | | | | | | |||||||
| | | 6/15/2017 | | | 77,084 | | | | | | | 18.09 | | | 6/14/2025 | | | | | | | | | |||||||
| | | 11/15/2017 | | | 87,500 | | | | | | | 43.07 | | | 11/14/2025 | | | | | | | | | |||||||
| | | 12/15/2017 | | | 37,625 | | | | | | | 56.90 | | | 12/14/2025 | | | | | | | | | |||||||
| | | 12/14/2018 | | | 48,400 | | | | | | | 36.51 | | | 12/13/2026 | | | | | | | | | |||||||
| | | 12/14/2018 | | | 48,400 | | | | | | | 36.51 | | | 12/13/2026 | | | | | | | | | |||||||
| | | 10/01/2019 | | | 118,750 | | | 31,250(5) | | | | | 18.43 | | | 9/30/2027 | | | | | | | | | ||||||
| | | 12/12/2019 | | | 53,662 | | | 17,888(5) | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | ||||||
| | | 12/12/2019 | | | 53,669 | | | 17,881(4) | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | ||||||
| | | 12/12/2019 | | | 15,075 | | | 5,025(5) | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | ||||||
| | | | | Option Awards | | | Stock Awards | |||||||||||||||||||||||
Name (a) | | | Grant Date | | | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | | | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#)(1) (d) | | | Option Exercise Price ($) (e) | | | Option Expiration Date(2) (f) | | | Number of Shares or Units of Stock That Have Not Vested (#) (g) | | | Market Value of Shares or Units of Stock That Have Not Vested ($)(3) (h) | | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (i) | | | Equity Incentive Plan Awards: Market or Payout value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(3) (j) |
| | | 12/18/2020 | | | 35,750 | | | 35,750(5) | | | | | 18.75 | | | 12/17/2028 | | | | | | | | | ||||||
| | | 12/18/2020 | | | | | 71,500(6) | | | | | 18.75 | | | 12/17/2028 | | | | | | | | | |||||||
| | | 12/18/2020 | | | | | | | | | | | | | 13,334(7) | | | 30,135 | | | | | ||||||||
| | | 12/18/2020 | | | | | | | | | | | | | | | | | 40,000(8) | | | 90,400 | ||||||||
| | | 12/16/2021 | | | 23,500 | | | 70,500(5) | | | | | 13.22 | | | 12/15/2029 | | | | | | | | | ||||||
| | | 12/16/2021 | | | | | | | 94,000(6) | | | 13.22 | | | 12/15/2029 | | | | | | | | | |||||||
| | | 12/16/2021 | | | | | | | | | | | | | 35,300(7) | | | 79,778 | | | | | ||||||||
| | | 12/16/2021 | | | | | | | | | | | | | | | | | 52,950(8) | | | 119,667 | ||||||||
| | | 12/16/2021 | | | | | | | | | | | | | | | | | 35,400(9) | | | 80,004 | ||||||||
| | | 08/15/2022 | | | 30,000 | | | 240,000(10) | | | | | 4.91 | | | 08/14/2030 | | | | | | | | | ||||||
| | �� | 08/15/2022 | | | | | | | 270,000(11) | | | 4.91 | | | 08/14/2030 | | | | | | | | | |||||||
| | | 08/15/2022 | | | | | | | | | | | | | 123,750(7) | | | 279,675 | | | | | ||||||||
| | | 08/15/2022 | | | | | | | | | | | | | | | | | 135,000(8) | | | 305,100 | ||||||||
| | | | | Option Awards | | | Stock Awards | |||||||||||||||||||||||
Name (a) | | | Grant Date | | | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | | | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) (d) | | | Option Exercise Price ($) (e) | | | Option Expiration Date(3) (f) | | | Number of Shares or Units of Stock That Have Not Vested (#) (g) | | | Market Value of Shares or Units of Stock That Have Not Vested ($)(4) (h) | | | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (i) | | | Equity Incentive Plan Awards: Market or Payout value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(4) (j) |
| Jonathan Zalevsky | | | 5/31/2016 | | | 37,500 | | | | | | | 15.44 | | | 5/30/2024 | | | | | | | | | ||||||
| | | 11/15/2016 | | | 46,875 | | | | | | | 13.93 | | | 11/14/2024 | | | | | | | | | |||||||
| | | 12/13/2016 | | | 15,500 | | | | | | | 12.24 | | | 12/12/2024 | | | | | | | | | |||||||
| | | 3/16/2017 | | | 21,250 | | | | | | | 15.71 | | | 3/14/2025 | | | | | | | | | |||||||
| | | 4/18/2017 | | | 36,459 | | | | | | | 18.585 | | | 4/17/2025 | | | | | | | | | |||||||
| | | 6/15/2017 | | | 77,084 | | | | | | | 18.09 | | | 6/14/2025 | | | | | | | | | |||||||
| | | 11/15/2017 | | | 87,500 | | | | | | | 43.07 | | | 11/14/2025 | | | | | | | | | |||||||
| | | 12/15/2017 | | | 37,625 | | | | | | | 56.90 | | | 12/14/2025 | | | | | | | | | |||||||
| | | 12/14/2018 | | | 48,400 | | | | | | | 36.51 | | | 12/13/2026 | | | | | | | | | |||||||
| | | 12/14/2018 | | | 48,400 | | | | | | | 36.51 | | | 12/13/2026 | | | | | | | | | |||||||
| | | 10/01/2019 | | | 150,000 | | | | | | | 18.43 | | | 9/30/2027 | | | | | | | | | |||||||
| | | 12/12/2019 | | | 71,550 | | | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | |||||||
| | | 12/12/2019 | | | 71,550 | | | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | |||||||
| | | 12/12/2019 | | | 20,100 | | | | | | | 21.79 | | | 12/11/2027 | | | | | | | | | |||||||
| | | 12/18/2020 | | | 53,625 | | | 17,875(5) | | | | | 18.75 | | | 12/17/2028 | | | | | | | | | ||||||
| | | 12/18/2020 | | | | | | | 71,500(6) | | | 18.75 | | | 12/17/2028 | | | | | | | | | |||||||
| | | 12/18/2020 | | | | | | | | | | | | | | | | | 40,000(8) | | | 22,800 | ||||||||
| | | 12/16/2021 | | | 47,000 | | | 47,000(5) | | | | | 13.22 | | | 12/15/2029 | | | | | | | | | ||||||
| | | 12/16/2021 | | | | | | | 94,000(6) | | | 13.22 | | | 12/15/2029 | | | | | | | | | |||||||
| | | 12/16/2021 | | | | | | | | | | | | | 17,650(7) | | | 10,061 | | | | | ||||||||
| | | 12/16/2021 | | | | | | | | | | | | | | | | | 52,950(8) | | | 30,182 | ||||||||
| | | 08/15/2022 | | | 120,000 | | | 150,000(9) | | | | | 4.91 | | | 08/14/2030 | | | | | | | | | ||||||
| | | 08/15/2022 | | | | | | | 270,000(10) | | | 4.91 | | | 08/14/2030 | | | | | | | | | |||||||
| | | 08/15/2022 | | | | | | | | | | | | | 78,750(7) | | | 44,888 | | | | | ||||||||
| | | 08/15/2022 | | | | | | | | | | | | | | | | | 135,000(8) | | | 76,950 | ||||||||
| | | 12/13/2023 | | | | | 250,000(9) | | | | | 0.50 | | | 12/12/2031 | | | | | | | | | |||||||
| | | 12/13/2023 | | | | | | | 250,000(10) | | | 0.50 | | | 12/12/2031 | | | | | | | | | |||||||
Jillian B. Thomsen(2) | | | 12/13/2016 | | | 37,500 | | | | | | | 12.24 | | | 6/16/2024 | | | | | | | | | ||||||
| | | 12/13/2016 | | | 37,500 | | | | | | | 12.24 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/15/2017 | | | 26,250 | | | | | | | 56.90 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/15/2017 | | | 26,250 | | | | | | | 56.90 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/14/2018 | | | 22,150 | | | | | | | 36.51 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/14/2018 | | | 22,150 | | | | | | | 36.51 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/12/2019 | | | 23,276 | | | | | | | 21.79 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/12/2019 | | | 23,275 | | | | | | | 21.79 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/12/2019 | | | 10,325 | | | | | | | 21.79 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/18/2020 | | | 17,279 | | | | | | | 18.75 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 12/16/2021 | | | 14,100 | | | | | | | 13.22 | | | 6/16/2024 | | | | | | | | | |||||||
| | | 8/15/2022 | | | 92,187 | | | | | | | 4.91 | | | 6/16/2024 | | | | | | | | | |||||||
| (1) |
| (2) | Ms. Thomsen served as our Chief Financial Officer and Chief Accounting Officer from July 1, 2022 to April 17, 2023, and her employment with the Company ended on June 16, 2023. All stock options and RSUs awarded to Ms. Thomsen that were unvested as of June 16, 2023, were forfeited. |
| (3) | For all NEOs, except for Ms. Thomsen, the expiration date shown is the normal expiration date occurring on the eighth anniversary of the grant date, which is the latest date that the stock options may be exercised. Stock options may terminate earlier in certain |
65
circumstances, such as in connection with an NEO’s termination of employment or in connection with certain corporate transactions, including a change of control. Pursuant to the terms of the Thomsen Separation Agreement, Ms. Thomsen’s vested stock options expire upon the earlier of (i) 12 months after her employment end date and (ii) the ordinary term expiration of the option.
| Restricted stock unit market value is calculated based on |
| (5) | The stock options vest pro-rata on a monthly basis over a period of four years from the date of grant, subject to the executive’s continued service through each vesting date. |
| (6) | The stock options vest only after achievement of specified performance criteria and pro-rata monthly vesting over a four-year period from the date of grant, subject to the executive’s continued service through each vesting date. |
| (7) | The RSUs vest pro-rata on a quarterly basis over a three-year period from the date of grant. |
| (8) | The RSUs vest only after achievement of specified performance criteria and pro-rata quarterly vesting over a three-year period from the date of grant, subject to the executive’s continued service through each vesting date. |
| (9) |
| The stock options vest pro-rata on a monthly basis over a period of three years from the date of grant, subject to the executive’s continued service through each vesting date. |
| The stock options vest only after achievement of specified performance criteria and pro-rata monthly vesting over a three-year period from the date of grant, subject to the executive’s continued service through each vesting date. |
The following table includes certain information with respect to the exercise of stock options and vesting of stock awards held by the NEOs during the fiscal year ended December 31, 2022.2023.
| | | Option Awards | | | Stock Awards | |||||||
Name (a) | | | Number of Shares Acquired on Exercise (#) (b) | | | Value Realized on Exercise ($) (c)(1) | | | Number of Shares Acquired on Vesting (#) (d) | | | Value Realized on Vesting ($) (e)(2) |
Howard W. Robin | | | — | | | — | | | 243,219 | | | 1,251,856 |
Jillian B. Thomsen | | | — | | | — | | | 47,151 | | | 232,340 |
Mark A. Wilson | | | — | | | — | | | 49,399 | | | 267,909 |
Jonathan Zalevsky, Ph.D. | | | — | | | — | | | 111,808 | | | 612,857 |
Gil M. Labrucherie3 | | | — | | | — | | | 70,233 | | | 429,739 |
John Northcott4 | | | — | | | — | | | 36,059 | | | 273,504 |
| | | Option Awards | | | Stock Awards | |||||||
| Name (a) | | | Number of Shares Acquired on Exercise (#) (b) | | | Value Realized on Exercise ($) (c)(1) | | | Number of Shares Acquired on Vesting (#) (d) | | | Value Realized on Vesting ($) (e)(2) |
| Howard W. Robin | | | — | | | — | | | 214,442 | | | 279,186 |
| Sandra Gardiner | | | — | | | — | | | — | | | — |
| Mark A. Wilson | | | — | | | — | | | 77,429 | | | 100,806 |
| Jonathan Zalevsky, Ph.D. | | | — | | | — | | | 75,984 | | | 98,925 |
Jillian B. Thomsen(3) | | | — | | | — | | | 33,857 | | | 65,572 |
| (1) | The value realized upon the exercise of stock options is calculated by (a) subtracting the stock option exercise price from the market price on the date of exercise to get the realized value per share, and (b) multiplying the realized value per share by the number of shares underlying the stock options exercised. |
| (2) | The value realized upon vesting of RSUs is calculated by multiplying the number of RSUs vested by the market price on the vest date. |
| (3) | Information provided up until |
The following section describes the benefits that may become payable to the NEOs in connection with their termination of employment with us or in connection with a change of control. We did not make any policy changes in 20222023 in connection with any terminations under our 20222023 Restructuring Plan. Please see “Compensation Discussion and Analysis—Severance and Change of Control Benefits” for a discussion of how the payments and benefits presented below were determined.
Severance Benefits—No Change of Control
Mr. Robin and, Ms. Thomsen are and, during theirher employment with the Company, Mr. Labrucherie and Mr. Northcott were,Ms. Thomsen, was, a party to certain letter agreement or our standard form executive employment agreement, and these agreements include provisions for severance benefits upon certain terminations of employment that are not related to a change of control. Upon a termination of employment by us without Cause or by the executive for a Good Reason Resignation (as defined in the CIC Plan and described below), the executive would be entitled to the following severance benefits: (i) a cash severance payment equal to his or her total annual cash compensation target (including base salary and the target value of his or her annual incentive bonus, as such bonus target may be adjusted downward to take into account our performance through the fiscal quarter preceding termination), (ii) an extension of the exercise period for the vested and unexercised portion of all outstanding stock options held by him or her for up to eighteen (18) months for Mr. Robin and twelve (12) months for Ms. Thomsen, Mr. Labrucherie and Mr. Northcott, following termination and
66
(iii) payment of all applicable COBRA premiums for the same period as the severance benefit following the termination date. In order to receive the severance benefits described above, each executive must first execute an effective waiver and release of claims in favor of us. Each executive’s cash severance payment would ordinarily be paid in a lump-sum within 60 days following the executive’s separation from service, although payment will be delayed to the extent required to comply with Section 409A of the Internal Revenue Code.
Neither Dr. Zalevsky nor Mr. Wilson is a party to a letter agreement or our standard form executive employment agreement that provides for severance benefits upon certain terminations of employment that are not related to a change of control. Upon a termination of employment by us without Cause or by the executive for a Good Reason Resignation (as defined in the CIC Plan and described below), Dr. Zalevsky and Mr. Wilson would be entitled to the following severance benefits: (i) a negotiated cash severance payment, (ii) an extension of the exercise period for the vested and unexercised portion of all outstanding stock options held by them for up to three (3) months following termination and (iii) payment of all applicable COBRA premiums for the same period
as the severance benefit following the termination date. In order to receive the severance benefit described above, Dr. Zalevsky and Mr. Wilson must first execute an effective waiver and release of claims in favor of us. Dr. Zalevsky’s and Mr. Wilson’s cash severance payment would ordinarily be paid in a lump-sum within 60 days following their separation from service, although payment will be delayed to the extent required to comply with Section 409A of the Internal Revenue Code.
If an NEO’s employment with us terminates due to death, the executive’s outstanding unvested stock options will become fully vested and will be exercisable for up to eighteen months following termination pursuant to the terms of the Company’s equity incentive compensation plans and agreement, and the NEO’s RSUs will become fully vested and released. If the termination due to death occurs before the end of the two year performance period for any TSR RSUs, the NEO will vest assuming target achievement. If the termination due to death occurs on or after the end of the two year performance period but prior to the Organization and Compensation Committee’s determination of the number of TSR RSUs credited following the end of the performance period, the NEO will vest in the number of TSR RSUs based on the Compensation Committee’s determination of the number of TSR RSUs credited following the end of the performance period. In addition, in the case of Mr. Robin, the executive’s estate would be entitled to a pro-rata portion of the target annual incentive bonus for the year in which their death occurred.
If an NEO terminates employment with us as a result of disability, vested stock options will be exercisable for up to twelve months following termination pursuant to the terms of the Company’s stock option agreement. For Mr. Robin, he is also entitled to have 50% of outstanding unvested stock options become fully vested upon disability for stock options granted under the equity plan in place at time of grant in accordance with the terms and conditions of his offer letter agreement. The NEO’s unvested RSUs are forfeited. In addition, pursuant to his offer letter agreement, Mr. Robin would be entitled to receive a pro-rata portion of his target annual incentive bonus for the year of termination in the event of a termination due to disability.
Pursuant to our standard form employment agreement, following a termination of employment, each NEO will be subject to an indefinite restriction on the disclosure of our confidential information and a one-year non-solicitation restriction covering our customers and employees.
The following table lists the estimated amounts that would become payable to each of the NEOs under the circumstances described above, assuming that the applicable triggering event occurred on December 31, 2022. With respect to Mr. Labrucherie and Mr. Northcott, the following table lists the actual payments made to each individual in connection with their termination from the Company.2023.
Executive & Triggering Event | | | Estimated Value of Cash Severance ($) | | | Estimated Value of COBRA Benefits ($)(1) | | | Estimated Value of Vesting Acceleration ($)(2) | | | Estimated Value of Pro-Rata Bonus ($) | | | Estimated Total ($) |
Howard W. Robin | | | | | | | | | | | |||||
Without Cause or Good Reason | | | 2,169,180 | | | 54,781 | | | 0 | | | 0 | | | 2,223,961 |
Disability | | | 0 | | | N/A | | | 0 | | | 1,084,590 | | | 1,084,590 |
Death | | | 0 | | | N/A | | | 2,765,372 | | | 1,084,590 | | | 3,849,962 |
Jillian B. Thomsen | | | | | | | | | | | |||||
Without Cause or Good Reason | | | 880,000 | | | 37,851 | | | 0 | | | 0 | | | 917,851 |
Disability | | | N/A | | | N/A | | | N/A | | | N/A | | | N/A |
Death | | | 0 | | | 0 | | | 878,950 | | | 0 | | | 878,950 |
Mark A Wilson(3) | | | | | | | | | | | |||||
Without Cause or Good Reason | | | N/A | | | N/A | | | N/A | | | N/A | | | N/A |
Disability | | | N/A | | | N/A | | | N/A | | | N/A | | | N/A |
Death | | | 0 | | | 0 | | | 1,004,378 | | | 0 | | | 1,004,378 |
Johnathan Zalevsky, Ph.D.(3) | | | | | | | | | | | |||||
Without Cause or Good Reason | | | N/A | | | N/A | | | N/A | | | N/A | | | N/A |
Disability | | | N/A | | | N/A | | | N/A | | | N/A | | | N/A |
Death | | | N/A | | | N/A | | | 984,759 | | | 0 | | | 984,759 |
Gil M. Labrucherie(4) | | | | | | | | | | | |||||
Voluntary Resignation | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 |
John Northcott(5) | | | | | | | | | | | |||||
Without Cause or Good Reason | | | 921,206 | | | 8,140 | | | 0 | | | 0 | | | 929,346 |
| Executive & Triggering Event | | | Estimated Value of Cash Severance ($) | | | Estimated Value of COBRA Benefits ($)(1) | | | Estimated Value of Vesting Acceleration ($)(2) | | | Estimated Value of Pro-Rata Bonus ($) | | | Estimated Total ($) |
| Howard W. Robin | | | | | | | | | | | |||||
| Without Cause or Good Reason | | | 2,169,180 | | | 61,971 | | | — | | | — | | | 2,231,151 |
| Disability | | | — | | | — | | | 338,348 | | | 1,084,590 | | | 1,422,938 |
| Death | | | — | | | — | | | 676,697 | | | 1,084,590 | | | 1,761,287 |
| Sandra Gardiner | | | — | | | — | | | — | | | — | | | — |
Mark A Wilson(3) | | | | | | | | | | | |||||
| Without Cause or Good Reason | | | N/A | | | N/A | | | N/A | | | N/A | | | N/A |
| Disability | | | — | | | — | | | — | | | — | | | — |
| Death | | | — | | | — | | | 231,025 | | | — | | | 231,025 |
Johnathan Zalevsky, Ph.D.(3) | | | | | | | | | | | |||||
| Without Cause or Good Reason | | | N/A | | | N/A | | | N/A | | | N/A | | | N/A |
| Disability | | | — | | | — | | | — | | | — | | | — |
| Death | | | — | | | — | | | 212,154 | | | — | | | 212,154 |
| (1) | The value of COBRA benefits are based upon actual rates as of December |
| (2) | For purposes of this table, we have assumed that (i) the price per share of our common stock is equal to the closing price per share on the last trading day of the fiscal year ended December |
| (3) | Neither Dr. Zalevsky nor Mr. Wilson is a party to a letter agreement or our standard form executive employment agreement that provides for severance benefits upon certain terminations of employment that are not related to a change of control. |
Severance Benefits—Change of Control
Each of the NEOs is covered under the CIC Plan. The CIC Plan provides for certain severance benefits to these executives and our other employees covered by the plan upon certain terminations of employment occurring in connection with a change of control of us.
If a change of control of the Company occurs, each NEO will be entitled to severance benefits under the CIC Plan if the executive’s employment is terminated by us or a successor company without Cause or by the executive for Good Reason Resignation (as defined in the CIC Plan), in each case within a period generally beginning on the date the agreement providing for a change of control is executed and ending twelve months following the change of control. Severance benefits under the CIC Plan include: (i) a cash severance payment equal to twelve (12) months of base salary (twenty-four (24) months for Mr. Robin) and the target value of the executive’s annual incentive bonus; (ii) payment by us of the same portion of the executive’s COBRA premiums as we pay for active employees’ group health coverage for up to twelve (12) months (eighteen (18) months for Mr. Robin) following termination; (iii) provision of up to $5,000 for outplacement services received within twelve (12) months following termination; (iv) accelerated vesting of all outstanding stock options and other
outstanding equity awards; and (v) other than in the case of Dr. Zalevsky, a “gross up” payment to compensate the executive for excise taxes (if any) on payments that are considered “parachute payments” under Section 280G of the Internal Revenue Code and therefore subject to an excise tax imposed under Section 4999 of the Code, but only to the extent the excise tax cannot be avoided by reducing the severance benefits by an amount not exceeding 10% such that the executive receives a greater-after tax amount as a result of the “cut-back” in benefits. In April 2011, the board of directors amended the CIC Plan so that this “gross up” benefit is not available for new hires following January 1, 2010 but is grandfathered for employees who joined the CIC Plan before that date so long as they are not promoted to a position such that he or she would be entitled to additional benefits under the plan. Accordingly, Dr. Zalevsky is not entitled to this “gross up” benefit as he joined the CIC Plan after January 1, 2010. In order to receive the severance benefits described above, the executive must first execute an effective waiver and release of claims in favor of us pursuant to a separation and release agreement. Each executive’s cash severance payment will ordinarily be paid in a lump-sum within
68
60 days following the executive’s separation from service, although payment will be delayed to the extent required to comply with Section 409A of the Internal Revenue Code.
For the purposes of the CIC Plan, a Good Reason Resignation means a resignation upon the occurrence of one or more of the following events: (i) assignment of any authority, duties or responsibilities that results in a material diminution in the executive’s authority, duties or responsibilities as in effect immediately prior to the change of control; (ii) assignment to a work location more than 50 miles from the executive’s immediately previous work location, unless such reassignment of work location decreases the executive’s commuting distance from his or her residence to the executive’s assigned work location; (iii) a material diminution in the executive’s monthly base salary as in effect on the date of the change of control or as increased thereafter; (iv) notice to the executive by us or the successor company during the 12-month period following the change of control that the executive’s employment will be terminated under circumstances that would trigger severance benefits under the CIC Plan but for the designation of a date for termination that is greater than 12 months following the change of control and (v) for Mr. Robin, if he does not serve in his same position in the successor company or is not appointed to the board of directors of the successor company. In order for a Good Reason Resignation to occur, the executive must first give us timely written notice of the grounds for good reason resignation, and we must have failed to cure such condition after a period of 30 days.
Pursuant to the CIC Plan, the separation and release agreement that each of the NEOs will be required to execute to receive severance benefits under the plan will also require each executive to agree to continue to be subject to the restrictions on the disclosure of our confidential information in his or her employment agreement, to non-solicitation restrictions and to certain other restrictions.
Had a change of control occurred (where outstanding equity awards were assumed, continued or substituted by a successor entity) during the 20222023 fiscal year and had the employment of each of the NEOs terminated on December 31, 20222023 under one of the qualifying circumstances described above, each executive would have been entitled to receive the estimated benefits set forth in the table below.
Name | | | Estimated Value of Cash Severance ($) | | | Estimated Value of Welfare and Outplacement Benefits ($)(1) | | | Estimated Value of Vesting Acceleration ($)(2) | | | Estimated Amount Forfeited by Executive(3) | | | Estimated Value of Excise Tax Gross-Up ($) | | | Estimated Total ($) |
Howard W. Robin | | | 4,338,360 | | | 173,529 | | | 2,765,372 | | | — | | | 0 | | | 7,277,261 |
Jillian B. Thomsen | | | 880,000 | | | 40,862 | | | 878,950 | | | — | | | 0 | | | 1,799,812 |
Mark A. Wilson | | | 864,000 | | | 41,846 | | | 1,004,378 | | | — | | | 0 | | | 1,910,224 |
Jonathan Zalevsky, Ph.D. | | | 1,125,584 | | | 19,246 | | | 984,759 | | | — | | | 0 | | | 2,129,588 |
| Name | | | Estimated Value of Cash Severance ($) | | | Estimated Value of Welfare and Outplacement Benefits ($)(1) | | | Estimated Value of Vesting Acceleration ($)(2) | | | Estimated Amount Forfeited by Executive(3) | | | Estimated Value of Excise Tax Gross-Up ($) | | | Estimated Total ($) |
| Howard W. Robin | | | 4,338,360 | | | 97,956 | | | $676,789 | | | 0 | | | 0 | | | 5,113,105 |
| Mark A. Wilson | | | 864,000 | | | 47,722 | | | $231,059 | | | 0 | | | 0 | | | 1,142,781 |
| Jonathan Zalevsky, Ph.D. | | | 1,125,584 | | | 17,280 | | | $212,186 | | | 0 | | | 0 | | | 1,355,050 |
| (1) | This amount includes estimated COBRA premiums based upon actual rates as of December |
| (2) | Pursuant to the terms of our equity compensation plans, these NEOs would also have been entitled to this same full equity acceleration (i) if a corporate transaction (as defined in the applicable plan) occurred and the surviving or acquiring corporation refused to assume outstanding equity awards or substitute similar replacement awards for outstanding equity awards or (ii) upon the acquisition by any person of beneficial ownership of 50% or more of the combined voting power of our shares in a transaction that is not a corporate transaction as defined in the applicable plan. For purposes of this table, we have assumed that (i) the price per share of our common stock is equal to the closing price per share on the last trading day of the fiscal year ended December |
| (3) | Executives with a gross-up provision are required to forfeit payments up to 10% if it will avoid an excise tax exposure. |
CEO Pay Ratio
Under the Dodd-Frank Wall Street Reform and Consumer Protection Act, we are required to disclose the median of the annual total compensation of our employees, the annual total compensation of our President and CEO Howard Robin, and the ratio of these two amounts.
We have estimated the median of the 20222023 annual total compensation of our employees, excluding Mr. Robin, to be $240,505.$216,654 as calculated in accordance with Item 402(U) of Regulation S-K. The annual total compensation of our President and CEO, as reported in the Summary Compensation Table – Fiscal 2020-20222021-2023 is $11,257,830.$3,135,576. The ratio of the annual total compensation of our President and CEO to the estimated median of the annual total compensation of our employees was 4714 to 1. We believe this pay ratio is a reasonable estimate calculated in a manner consistent with SEC rules.
69
We selected December 31, 20222023 as the date to identify our median employee. We determined our median employee based on the sum of taxable wages for 2022,2023, FASB ASC Topic 718 value of option and stock awards granted in 2022,2023, and other compensation including taxable benefits of each of our employees, excluding Mr. Robin, earned in 2022.2023.
PAY VERSUS PERFORMANCE
In accordance with rules adopted by the Securities and Exchange CommissionSEC pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, we provide the following disclosure regarding executive compensation for our principal executive officer (“PEO”) and Non-PEO NEOs and Company performance for the fiscal years listed below. The Organization and Compensation Committee did not consider the pay versus performance disclosure below in making its pay decisions for any of the years shown.
| | Year | | | Summary Compensation Table Total for Howard W. Robin(1) ($) | | | Compensation Actually Paid to Howard W. Robin(1),(2),(3) ($) | | | Average Summary Compensation Table Total for Non-PEO NEOs(1) ($) | | | Average Compensation Actually Paid to Non-PEO NEOs(1),(2),(3) ($) | | | Value of Initial Fixed $100 Investment based on:(4) | | | Net Income ($ Millions) | | | Relative TSR Percentile(5) | | |||
| | TSR ($) | | | Peer Group TSR ($) | | |||||||||||||||||||||
| | (a) | | | (b) | | | (c) | | | (d) | | | (e) | | | (f) | | | (g) | | | (h) | | | (i) | |
| | 2022 | | | 11,275,830 | | | (8,738,814) | | | 3,091,330 | | | (3,426,583) | | | 10.47 | | | 113.65 | | | (368) | | | 9th | |
| | 2021 | | | 11,153,616 | | | 8,032,023 | | | 4,377,734 | | | 3,290,039 | | | 62.59 | | | 126.45 | | | (524) | | | 52nd | |
| | 2020 | | | 11,241,149 | | | 6,456,336 | | | 4,379,031 | | | 2,020,372 | | | 78.76 | | | 126.42 | | | (444) | | | 30th | |
| | Year | | | Summary Compensation Table Total for PEO1 ($) | | | Compensation Actually Paid to PEO1,2,3 ($) | | | Average Summary Compensation Table Total for Non-PEO NEOs1 ($) | | | Average Compensation Actually Paid to Non-PEO NEOs1,2,3 ($) | | | Value of Initial Fixed $100 Investment based on:4 | | | Net Income ($ Millions) | | | Relative TSR (Percentile Rank)5 | | |||
| | TSR ($) | | | Peer Group TSR ($) | | |||||||||||||||||||||
| | 2023 | | | 3,135,576 | | | (773,200) | | | 1,122,473 | | | (2,644) | | | 2.62 | | | 118.87 | | | (276) | | | 7th | |
| | 2022 | | | 11,275,830 | | | (8,738,814) | | | 3,091,330 | | | (3,426,582) | | | 10.47 | | | 113.65 | | | (368) | | | 9th | |
| | 2021 | | | 11,153,616 | | | 8,032,023 | | | 4,377,734 | | | 3,290,039 | | | 62.59 | | | 126.45 | | | (524) | | | 52nd | |
| | 2020 | | | 11,241,149 | | | 6,456,336 | | | 4,379,031 | | | 2,020,372 | | | 78.76 | | | 126.42 | | | (444) | | | 30th | |
| Howard W. Robin was our PEO for each year presented. The individuals comprising the Non-PEO NEOs for each year presented are listed below. |
| | 2020 | | | 2021 | | | 2022 | | 2020-2021 | | | 2022 | | | 2023 | |
| | Gil M. Labrucherie | | | Gil M. Labrucherie | | | Gil M. Labrucherie | | Gil M. Labrucherie | | | Gil M. Labrucherie | | | Jillian B. Thomsen | |
| | Mark A. Wilson | | | Mark A. Wilson | | | Jillian Thomsen | | Mark A. Wilson | | | Jillian B. Thomsen | | | Sandra Gardiner | |
| | Jonathan Zalevsky | | | Jonathan Zalevsky | | | Mark A. Wilson | | Jonathan Zalevsky | | | Mark A. Wilson | | | Mark A. Wilson | |
| | John Northcott | | | John Northcott | | | Jonathan Zalevsky | | John Northcott | | | Jonathan Zalevsky | | | Jonathon Zalevsky | |
| | | | | | John Northcott | | | | John Northcott | | | |
| The amounts shown for Compensation Actually Paid have been calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually earned, realized, or received by the Company’s NEOs. These amounts reflect the Summary Compensation Table Total with certain adjustments as described in footnote 3 below. |
| Compensation Actually Paid reflects the exclusions and inclusions of certain amounts for the PEO and the Non-PEO NEOs as set forth below. Equity values are calculated in accordance with FASB ASC Topic 718. Amounts in the Exclusion of Stock Awards and Option Awards column are the |
| | Year | | | Summary Compensation Table Total for Howard W. Robin ($) | | | Exclusion of Stock Awards and Option Awards for Howard W. Robin ($) | | | Inclusion of Equity Values for Howard W. Robin ($) | | | Compensation Actually Paid to Howard W. Robin ($) | | Year | | | Summary Compensation Table Total for PEO ($) | | | Exclusion of Stock Awards and Option Awards for PEO ($) | | | Inclusion of Equity Values for PEO ($) | | | Compensation Actually Paid to PEO ($) | |
| | 2022 | | | 11,275,830 | | | (9,441,747) | | | (10,572,897) | | | (8,738,814) | | 2023 | | | 3,135,576 | | | (927,160) | | | (2,981,616) | | | (773,200) | |
| | 2021 | | | 11,153,616 | | | (9,018,286) | | | 5,896,693 | | | 8,032,023 | | ||||||||||||||
| | 2020 | | | 11,241,149 | | | (9,000,374) | | | 4,215,561 | | | 6,456,336 | | ||||||||||||||
| | Year | | | Average Summary Compensation Table Total for Non-PEO NEOs ($) | | | Average Exclusion of Stock Awards and Option Awards for Non-PEO NEOs ($) | | | Average Inclusion of Equity Values for Non-PEO NEOs ($) | | | Average Compensation Actually Paid to Non-PEO NEOs ($) | | Year | | | Average Summary Compensation Table Total for Non-PEO NEOs ($) | | | Average Exclusion of Stock Awards and Option Awards for Non- PEO NEOs ($) | | | Average Inclusion of Equity Values for Non-PEO NEOs ($) | | | Average Compensation Actually Paid to Non-PEO NEOs ($) | |
| | 2022 | | | 3,091,330 | | | (2,147,011) | | | (4,370,902) | | | (3,426,583) | | 2023 | | | 1,122,473 | | | (102,523) | | | (1,022,594) | | | (2,644) | |
| | 2021 | | | 4,377,734 | | | (3,246,995) | | | 2,159,300 | | | 3,290,039 | | ||||||||||||||
| | 2020 | | | 4,379,031 | | | (3,212,381) | | | 853,722 | | | 2,020,372 | | ||||||||||||||
The amounts in the Inclusion of Equity Values in the tables above are derived from the amounts set forth in the following tables:
| | Year | | | Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for Howard W. Robin ($) | | | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for Howard W. Robin ($) | | | Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for Howard W. Robin ($) | | | Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for Howard W. Robin ($) | | | Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for Howard W. Robin ($) | | | Total - Inclusion of Equity Values for Howard W. Robin ($) | | Year | | | Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for PEO ($) | | | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for PEO ($) | | | Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for PEO ($) | | | Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for PEO ($) | | | Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for PEO ($) | | | Total - Inclusion of Equity Values for PEO ($) | |
| | 2022 | | | 3,767,091 | | | (10,961,983) | | | 323,187 | | | (3,020,288) | | | (680,904) | | | (10,572,897) | | 2023 | | | 1,071,594 | | | (3,517,124) | | | — | | | (536,086) | | | — | | | (2,981,616) | |
| | 2021 | | | 9,295,631 | | | (3,098,368) | | | — | | | (300,570) | | | — | | | 5,896,693 | | ||||||||||||||||||||
| | 2020 | | | 7,998,651 | | | (3,330,924) | | | — | | | (452,166) | | | — | | | 4,215,561 | | ||||||||||||||||||||
| | Year | | | Average Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for Non-PEO NEOs ($) | | | Average Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for Non-PEO NEOs ($) | | | Average Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for Non-PEO NEOs ($) | | | Average Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for Non-PEO NEOs ($) | | | Average Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for Non-PEO NEOs ($) | | | Total - Average Inclusion of Equity Values for Non-PEO NEOs ($) | | Year | | | Average Year- End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for Non- PEO NEOs ($) | | | Average Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for Non-PEO NEOs ($) | | | Average Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for Non- PEO NEOs ($) | | | Average Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for Non-PEO NEOs ($) | | | Average Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for Non-PEO NEOs ($) | | | Total - Average Inclusion of Equity Values for Non-PEO NEOs ($) | |
| | 2022 | | | 856,627 | | | (1,982,867) | | | 73,492 | | | (729,142) | | | (2,589,012) | | | (4,370,902) | | 2023 | | | 118,494 | | | (633,627) | | | — | | | (104,218) | | | (403,243) | | | (1,022,594) | |
| | 2021 | | | 3,346,858 | | | (1,258,934) | | | — | | | 71,376 | | | — | | | 2,159,300 | | ||||||||||||||||||||
| | 2020 | | | 2,849,255 | | | (1,668,738) | | | — | | | (326,795) | | | — | | | 853,722 | | ||||||||||||||||||||
| The Peer Group TSR set forth in this table utilizes the NASDAQ Biotechnology Index, which we also utilize in the stock performance graph required by Item 201(e) of Regulation S-K included in our |
| We determined Relative TSR |
The following chart sets forth the relationship between Compensation Actually Paid to our PEO, the average of Compensation Actually Paid to our Non-PEO NEOs, and the Company’s cumulative TSR over the threefour most recently completed fiscal years.years to that of the NASDAQ Biotechnology Index over the same period.
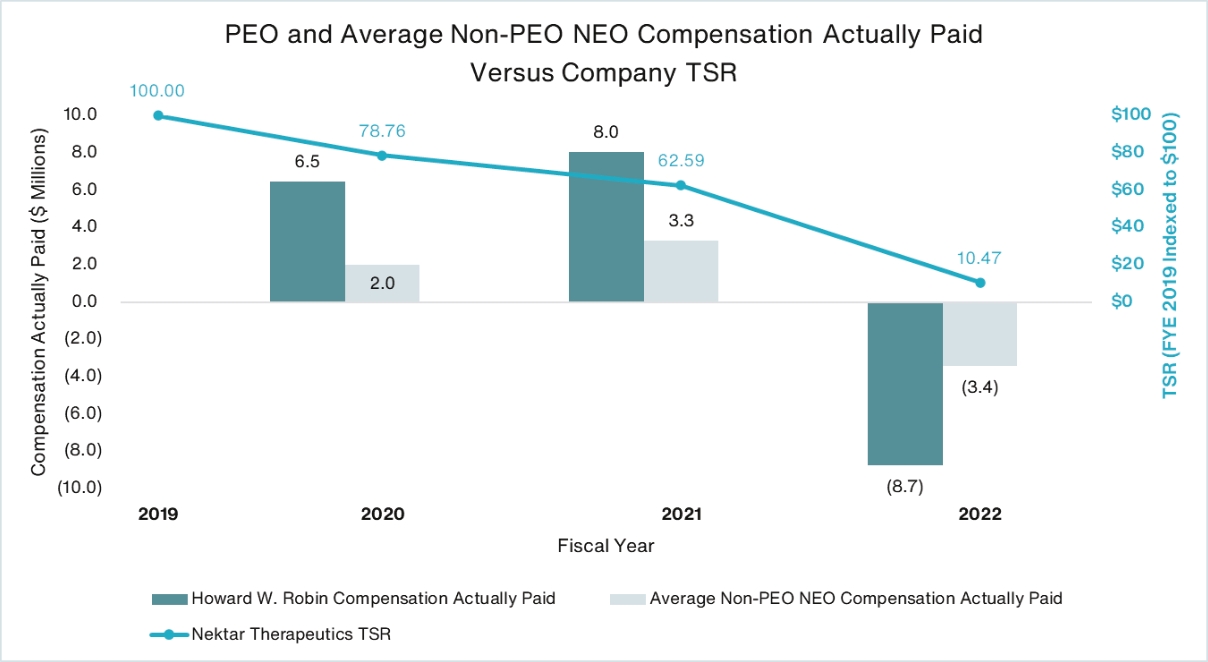

The following chart sets forth the relationship between Compensation Actually Paid to our PEO, the average of Compensation Actually Paid to our Non-PEO NEOs, and our Net Income during the threefour most recently completed fiscal years.
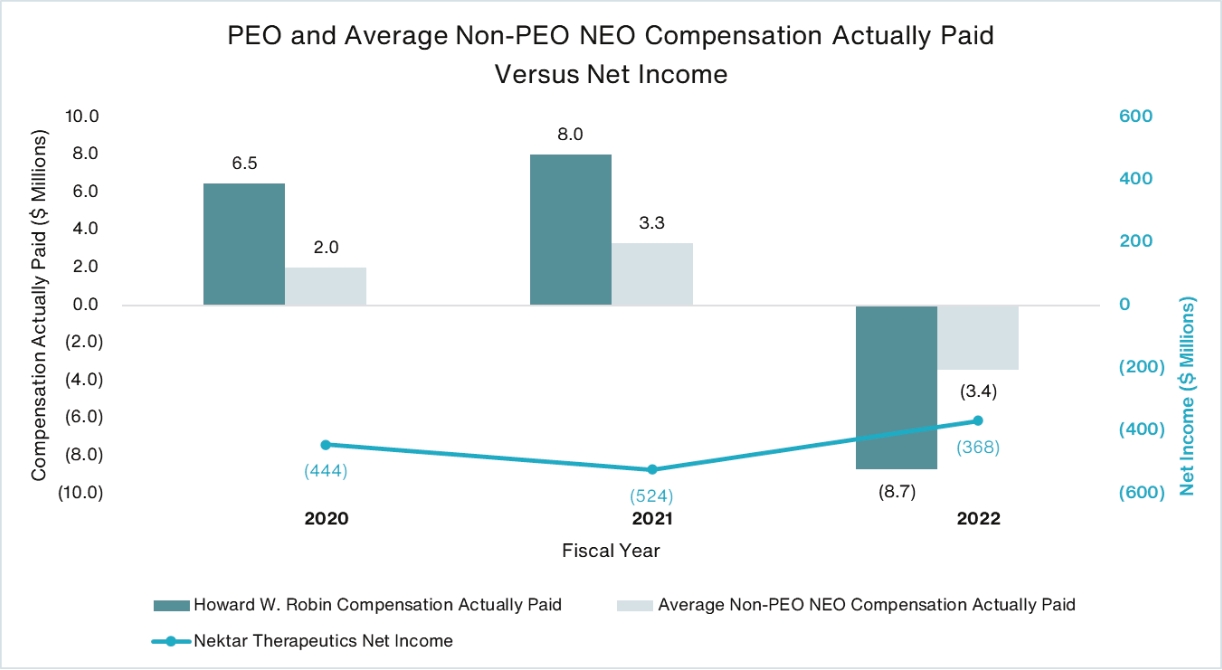

73
The following chart sets forth the relationship between Compensation Actually Paid to our PEO, the average of Compensation Actually Paid to our Non-PEO NEOs, and our Relative TSR Percentile(Percentile Rank) during the threefour most recently completed fiscal years.
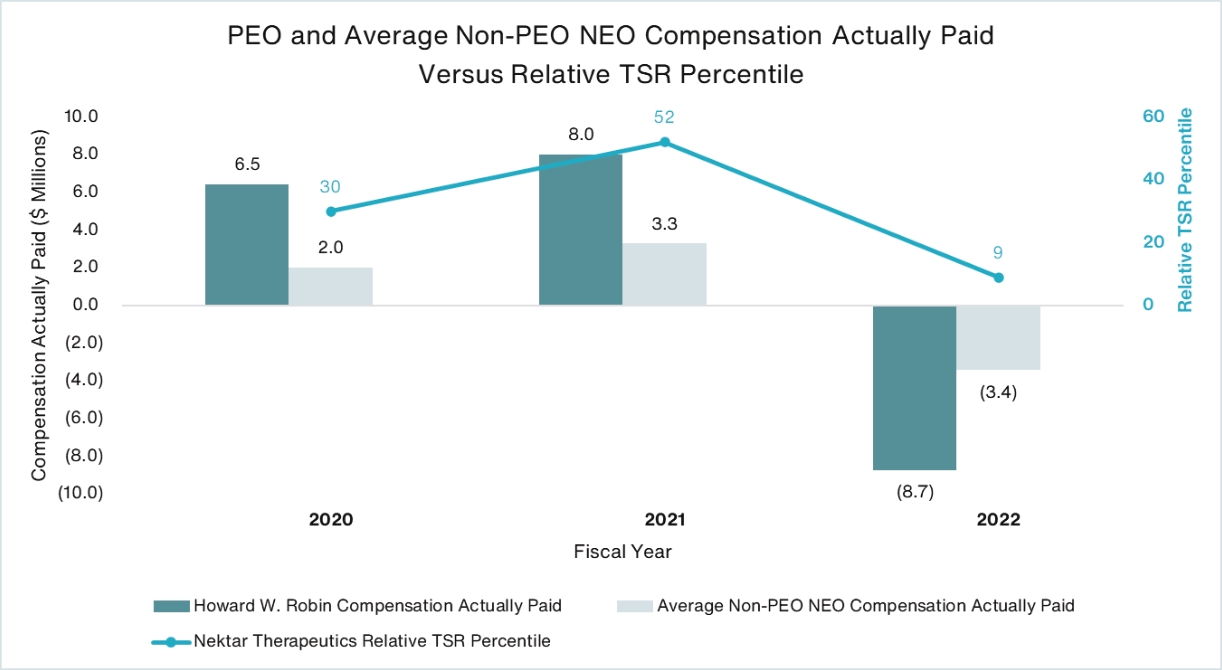
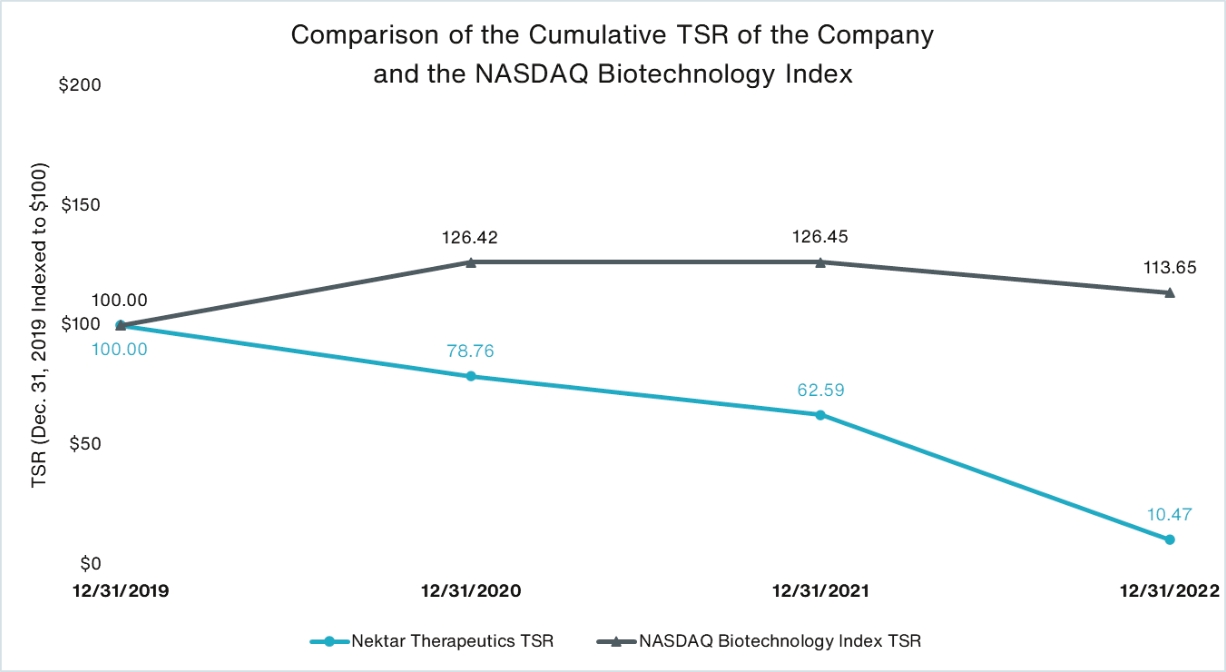

We believe that our compensation structure supports our business strategy while successfully aligning executive focus and interests with those of the Company and our stockholders. We seek to incentivize long-term performance and achievement of milestones that are important to building sustained success for the Company.
Our compensation structure includes several elements that collectively provide an effective compensation strategy that supports our philosophy of pay for performance. As discussed in the Compensation Discussion and Analysis section, the performance-based component of our NEO’s compensation is tied to the achievement of critical objective, performance milestones and annual corporate goals, which we believe provides strong performance incentives to our executives. For 2022,2023, we did not link any other financial performance measures to the Compensation Actually Paid to our PEO and Non-PEO NEOs other NEOS.than relative TSR.
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FEES AND SERVICES
The following table represents aggregate fees billed to us for fiscal years ended December 31, 20222023 and December 31, 20212022 by Ernst & Young LLP, our independent registered public accounting firm.
| | | Fiscal Year Ended | ||||
| | | 2022 | | | 2021 | |
Audit Fees | | | $2,087,610 | | | $1,999,527 |
Audit Related Fees | | | — | | | — |
Tax Fees | | | — | | | 12,853 |
All Other Fees | | | 3,245 | | | 4,465 |
Total | | | $2,090,855 | | | $2,016,845 |
| | | Fiscal Year Ended | ||||
| | | 2023 | | | 2022 | |
| Audit Fees | | | $1,901,271 | | | $2,087,610 |
| Audit Related Fees | | | — | | | — |
| Tax Fees | | | — | | | — |
| All Other Fees | | | 6,147 | | | 3,245 |
| Total | | | $1,907,418 | | | $2,090,855 |
Audit Fees. This category consists of fees related to the audit of our annual consolidated financial statements and our internal control over financial reporting, review of interim condensed consolidated financial statements included in our quarterly reports on Form 10-Q, and services that are normally provided by the independent registered public accounting firm in connection with statutory audit, registration statements and other regulatory filings.
Audit Related Fees. This category consists of fees related to assurance and related services by the independent registered public accounting firm that are reasonably related to the performance of the audit or review of our financial statements and are not reported under Audit Fees above.
Tax Fees. This category consists of fees related to services provided for international tax compliance and tax consultation services.
All Other Fees. This category consists of fees related to accessing Ernst & Young LLP’s online research database in 20212022 and 2022.2023.
The Audit Committee approved all fees described above.
The Audit Committee has adopted policies and procedures for the pre-approval of audit and non-audit services rendered by our independent registered public accounting firm, Ernst & Young LLP. The policy generally requires pre-approval for specified services in the defined categories of audit services, audit-related services and tax services up to specified amounts. Pre-approval may also be given as part of the Audit Committee’s approval of the scope of the engagement of the independent registered public accounting firm or on an individual explicit case-by-case basis before the independent registered public accounting firm is engaged to provide each service. The pre-approval of services may be delegated to one or more of the Audit Committee’s members, but the decision must be reported to the full Audit Committee at its next scheduled meeting.
Prior to Ernst & Young LLP rendering services other than audit services, the Audit Committee would review and approve such non-audit services only if such services were compatible with maintaining Ernst & Young LLP’s status as our independent registered public accounting firm.
The material in this report is being furnished and shall not be deemed “filed” with the SEC for purposes of Section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall the material in this section be deemed to be “soliciting material” or incorporated by reference in any registration statement or other document filed with the SEC under the Securities Act or the Exchange Act, except as otherwise expressly stated in such filing.
The Audit Committee is currently comprised of fourthree non-employee directors, Karin Eastham,R. Scott Greer, the Chairperson of the committee, R. Scott Greer, Jeff Ajer, and Roy A. Whitfield. Our board of directors determined that Mr. Greer, Mr. Ajer, and Mr. Whitfield and Ms. Eastham meet the independence requirements set forth in Rule 10A-3(b)(1) under the Exchange Act and in the applicable NASDAQ rules. In addition, the board of directors determined that Ms. Eastham and Mr. Greer qualify as Audit Committee financial experts as defined by SEC rules. The Audit Committee has the responsibility and authority described in the Nektar Therapeutics Audit Committee Charter, which has been approved by the board of directors. A copy of the Audit Committee Charter is available on our website at www.nektar.com.
The Audit Committee is responsible for assessing the information provided by management and our independent registered public accounting firm in accordance with its business judgment. Management is responsible for the preparation, presentation and integrity of our financial statements and for the appropriateness of the accounting principles and reporting policies that are used. Management is also responsible for testing the system of internal controls and reports to the Audit Committee on any deficiencies found. Our independent registered public accounting firm, Ernst & Young LLP, is responsible for auditing the annual financial statements and for reviewing the unaudited interim financial statements.
In fulfilling its oversight responsibilities, the Audit Committee has reviewed and discussed the audited financial statements in the annual report on Form 10-K for the year ended December 31, 20222023 with both management and our independent registered public accounting firm. The Audit Committee’s review included a discussion of the quality and integrity of the accounting principles, the reasonableness of significant estimates and judgments and the clarity of disclosures in the financial statements.
The Audit Committee reviewed with our independent registered public accounting firm the overall scope and plan of the audit. In addition, it met with our independent registered public accounting firm, with and without management present, to discuss the results of our independent registered public accounting firm’s examination, the evaluation of our system of internal controls, the overall quality of our financial reporting and such other matters as are required to be discussed under generally accepted accounting standards in the United States. The Audit Committee has also received from, and discussed with, our independent registered public accounting firm the matters required to be discussed by Auditing Standard No. 16, “Communications with Audit Committees” issued by the Public Company Accounting Oversight Board (“PCAOB”).
The Audit Committee has discussed with Ernst & Young LLP that firm’s independence from management and our Company, including the matters in the written disclosures and the letter regarding independence from Ernst & Young LLP required by applicable requirements of the PCAOB. The Audit Committee has also considered the compatibility of audit related and tax services with the auditors’ independence. Based on its evaluation, the Audit Committee has selected Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2023.2024.
In reliance on the reviews and discussions referred to above, the Audit Committee recommended to the board of directors, and the board of directors approved, the inclusion of the audited financial statements and management’s assessment of the effectiveness of our internal controls over financial reporting in the annual report on Form 10-K for the year ended December 31, 20222023 filed with the SEC.
Audit Committee
R. Scott Greer – Chairperson
Jeff Ajer
Roy A. Whitfield
The board of directors knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
Our website address is http://www.nektar.com. The information in, or that can be accessed through, our website is not deemed to be incorporated by reference into this proxy statement. Our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports are available, free of charge, on or through our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding our filings at www.sec.gov. In addition, a copy of our Annual Report on Form 10-K for the fiscal year ended December 31, 20222023 filed with the SEC is available without charge upon written request to: Secretary, Nektar Therapeutics, 455 Mission Bay Boulevard South, San Francisco, California 94158.
| | | By Order of the Board of Directors | |
| | | ||
| | | /s/ Mark A. Wilson | |
| | | Mark A. Wilson | |
| | | Senior Vice President, Chief Legal Officer and Secretary |
April 28, 202326, 2024
Exhibit A
NEKTAR THERAPEUTICS
AMENDMENT TO
AMENDED AND RESTATED
2017 PERFORMANCE INCENTIVE PLAN
In accordance with the provisions of the Nektar Therapeutics Amended and Restated 2017 Performance Incentive Plan (as amended from time to time, the “Plan”), the Plan is hereby amended as follows:
| 1. | Section 4.2(1) of the Plan is hereby deleted in its entirety and replaced with the following: |
“51,200,00059,200,000 shares of Common Stock, less”
| 2. | Section 4.2(a) of the Plan is hereby deleted in its entirety and replaced with the |
“The maximum number of shares of Common Stock that may be delivered pursuant to options qualified as incentive stock options granted under this Plan is 51,200,00059,200,000 shares.
| 3. | Except as modified herein, the Plan is not modified in any respect and remains in full force and effect. |
Approved by the Board of Directors: March 29, 202327, 2024
Approved by the Stockholders: June Juen __ , 20232024
A-1
0000906709 nktr:FairValueAtLastDayOfPriorYearOfEquityAwardsForfeitedDuringYearMember ecd:NonPeoNeoMember 2023-01-01 2023-12-31